The Brain: The Central Organ of Stress and Adaptation Across the Lifecourse
Bruce S. McEwen, PhD
The Rockefeller University, USA
Introduction
The important topics discussed by Gunnar, Herrera, Hostinar and by Heim rely upon a basic knowledge of brain-body interactions over the lifecourse. That is, effects of stress early in life clearly have a lasting effect on later mental and physical processes, increasing the risk for both mood and anxiety disorders, as well as cardiovascular and other systemic diseases. As a result of the recent progress of modern neuroscience and medicine, there is a growing understanding of brain-body interactions that underlie adaptation to stress and the accumulated pathophysiology that is associated with excessive and prolonged stress. Among the important concepts that have emerged is the notion that the brain is the central organ of stress because it regulates the major systems involved in adaptation and pathophysiology and is itself influenced both structurally and functionally by those systems. As summarized by both Gunnar et al.1 and by Heim,2 these effects begin early in life. Another major concept is that of “allostasis” and “allostatic overload,” reflecting the protective and damaging effects of the mediators of stress and adaptation and cumulative change resulting from prolonged stress and the lifestyle and behaviours associated with chronic stress. Related to this is the concept of biological embedding, namely, that those early life influences “get under the skin” and increase the impact of the cumulative aspects of prolonged stress and lifestyle.3
Subject
Research has made progress in understanding the role of the brain as the central organ of stress. Indeed, the brain is the key organ of the adaptive and maladaptive responses to stress because it determines what is threatening and, therefore, potentially stressful, as well as initiating the behavioural and many of the physiological responses to the stressors, which can be either adaptive or damaging.4,5 Stress involves two-way communication between the brain and the cardiovascular, immune and metabolic system via the autonomic nervous system and via endocrine mechanisms. The effects of stress involve measurements of multiple endpoints related to mediators of stress and adaptation and cumulative change in the body and brain.
Problems
The mediators of stress and adaptation operate in a non-linear manner (Figure 1) meaning that many of these mediators regulate each other in both positive and negative ways and also operate in a "U" shaped manner that is now referred to by the term hormesis.7 Beyond the “flight or fight” response to acute stress, there are events in daily life, including the individual life style, that produce a type of chronic stress and lead over time to wear and tear on the body (“allostatic overload”). Yet, the hormones and other mediators associated with stress and adaptation protect the body in the short-run and promote adaptation (“allostasis”).4,5,8 These systems are regulated by the brain via the hypothalamus and outputs via the autonomic and neuroendocrine systems. Input to the hypothalamus involves brain areas, such as the amygdala, the hippocampus and the prefrontal cortex, and these brain areas, along with the hypothalamus, respond to hormonal signals.
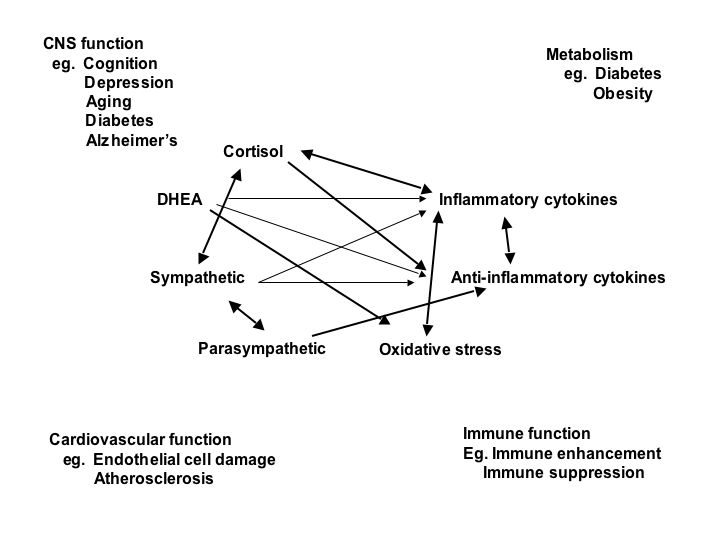
Figure 1. Non-linear network of mediators of allostasis involved in the stress response. Arrows indicate that each system regulates the others in a reciprocal manner, creating a non-linear network. Moreover, there are multiple pathways for regulation – e.g. inflammatory cytokine production is negatively regulated via anti-inflammatory cytokines, and via parasympathetic and glucocorticoid pathways, whereas sympathetic activity increases inflammatory cytokine production. Parasympathetic activity, in turn, modulates and limits sympathetic activity. Furthermore, mediators, such as cortisol and inflammatory cytokines, produce biphasic effects that are described now by the term hormesis (see text). Reprinted from McEwen6 by permission.
Research Context
The assessment of allostasis and allostatic overload is based upon collection of clinical information for the multiple systems involved in stress and adaptation; namely, the Hypothalamic-Pituitary-Adrenocorticol (HPA) axis, the autonomic nervous system, and metabolic parameters.9,10-12 Brain mechanisms involved in allostasis and allostatic overload can be studied in animal models using methods of modern neuroscience and translated to human subjects by brain imaging techniques that are rapidly developing.13
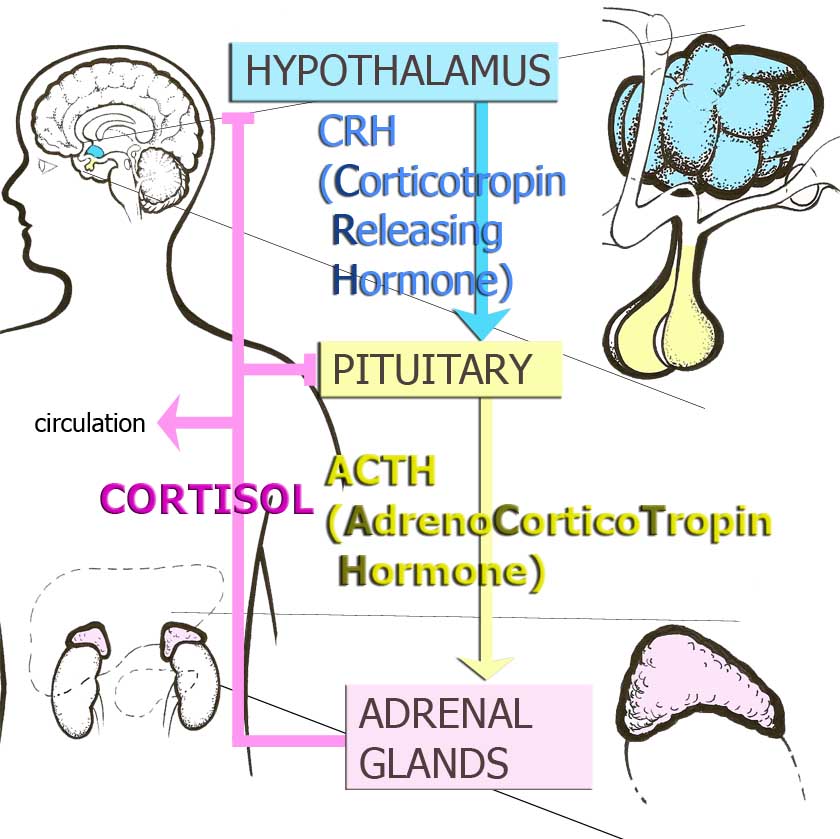
Figure 2. Hypothalamic-Pituitary Adrenocortical Axis (HPA) or Stress Hormone Axis.
Key Research Questions
Experiences involving social interactions and events in the physical environment are processed by the brain and are usually referred to under the rubric of “stress.” We now know, from animal models, that the brain changes in structure and function with experiences, including those of chronic stress, and that these changes in brain represent “adaptive plasticity,” in that they are largely reversible and appropriate for the conditions that cause them.5 With the exciting advances in neuroimaging, the living human brain can now be studied in some detail as it responds to stressful experiences during the life course, as well as how its structure and functions relate to physiologic states in the body.
Recent Research Results
Animal models have provided insights into how the brain responds to stress.5 The brain is a target of stress and the hippocampus was the first brain region, besides the hypothalamus, to be recognized as a target of glucocorticoids. Stress and stress hormones produce both adaptive and maladaptive effects on this brain region throughout the life course. Early life events influence lifelong patterns of emotionality and stress responsiveness and alter the rate of brain and body aging. The amygdala and prefrontal cortex, as well as the hippocampus, undergo stress-induced structural remodeling, which alters behavioural and physiological responses, including anxiety, aggression, mental flexibility, memory and other cognitive processes; glucocorticoids play a role in this remodeling along with excitatory amino acids, metabolic hormones and other intracellular and extracellular mediators.5
Human brain structural imaging has begun to reveal how the human hippocampus changes with experience. Recent evidence includes the relationship of 20 years of elevated perceived stress to reduced hippocampal volume,14,and how the hippocampus shrinks in disease states, such as Cushing’s disease, major depression, diabetes and posttraumatic stress disorder (PTSD)15,16 and pre-disease conditions, such as resulting from chronic jet-lag17 and elevated circulating inflammatory cytokines.18 Hippocampal volume is also smaller in both young and older people with low self esteem, accompanied by elevated HPA activity and lack of habituation to repeated stress.19
Based on animal models, and as noted above, the mechanisms for these changes are complex and are likely to involve not only glucocorticoids, but other hormones and mediators. Furthermore, physical activity and fitness in elderly subjects is associated with greater hippocampal volume and better memory function,20 just as greater activation of prefrontal cortical activity is associated with fitness and regular exercise and leads to better executive function.21,22
The prefrontal cortex, which is reversibly, functionally impaired by increased levels of perceived stress in medical students studying for the board exam23 is smaller in major depression24 and is smaller in people who self-report lower socioeconomic status.25 Functional activation of the prefrontal cortex is related to blood pressure responses,26 whereas functional activation in the amygdala is related to the negative response to fearful faces,27 which is exaggerated in people with early life adversity.28 Elevated amygdala functional activity is also related to the development of atherosclerosis.29
Animal models teach us that experiences, including stress-induced changes in brain structure, are largely reversible and that resilience in both brain structure and behaviour is the name of the game in adapting to changing environments.5 A corollary of this is that failure to show resilience is a feature of maladaptation and pathophysiology, including anxiety and depressive disorders and the downstream effects that these have on the rest of the body via the autonomic, neuroendocrine and immune systems. But how plastic is the human brain in response to interventions that effectively treat disorders that affect the brain as well as the rest of the body?
Although there is limited information, a few studies have shown longitudinally, in the same subjects, changes, for example, in functional activity30 and prefrontal cortex (PFC) structure31 in patients who successfully responded to behavioural therapy treatment for obsessive–compulsive disorder OCD and chronic fatigue, respectively. Another, albeit cross-sectional, study reports thicker cortical volume in right anterior insula and prefrontal cortex of subjects who had meditated for many years compared to matched controls.32 It is well known that, as an adjunct to pharmaceutical therapy, social and behavioural interventions, including regular physical activity and social support, are able to reduce the chronic stress burden and benefit brain and body health and resilience.5 Therefore, studies of how the brain is changed by behavioural, as well as by pharmaceutical therapies, are important future applications of brain imaging.
Research Gaps
Experience tells us that the social and physical environments in which people live and work have a huge effect upon psychological states. The nature of these environments also affects physical and mental health and risk for disease. Yet the scientific study of this important topic has been frustrated and fragmented by disciplinary boundaries between such fields as environmental toxicology, social psychology, sociology, health psychology, economics, epidemiology, psychiatry, pediatrics, neurology and medicine. As a result, only some of the considerable knowledge has penetrated, albeit inconsistently, into the mainline of medical teaching and practice, and neuroscience has been largely out of the picture until recently. As a result, a coherent conceptual framework has been missing, because the brain has not been fully recognized as playing a central role in physiological adaptation and the effects of stress, as well as being a target of stress and related behaviors.5 This is beginning to change with the translation of animal research findings to the human being via brain imaging techniques that are summarized above.
With brain imaging, most of the information has come from cross sectional studies, which can be only suggestive as to causality. With the advent of interventions that improve brain function and treat behavioural disorders, longitudinal studies of brain structure and function are not only possible, but essential, to show causality. As noted above, the best example thus far is that of the beneficial effects of physical activity. Another important area is that of the brain effects of Type 2 diabetes, noted above, but an important gap that must be filled is that such studies be carried out in the context of the developing brain and the consequences of Type 2 diabetes in childhood.
Conclusions
The lasting effects of stress on the body beginning early in life must be considered in the context of the whole lifecourse and the central role of the brain in the protective and damaging effects of the physiological mediators of stress and adaptation. The powerful effects of early life stress on the brain are beginning to be understood from animal models, as well as some brain imaging studies.33 These are now being considered in relation to measures of the mediators of allostasis and allostatic overload,34 since, as summarized above, circulating stress and metabolic hormones have important effects on the brain. In relation to the papers by Gunnar et al.1 and by Heim,2 it is possible to envision heroic life-long longitudinal studies of the brain beginning early in life, but perhaps more realistic to imagine shorter term studies of the effects of adversity on brain development in parallel with cognitive and physiological measures patterned after recent studies of a more limited nature.34,35 However, it would be even more valuable to follow longitudinally the effects of interventions designed to ameliorate effect of early life adversity, based, for example, on the nurse-family partnership program.a
Implications
Brain-body interactions are strongly influenced by the social and physical environments in which we live, and these are, in part, the products of practices and policies of private enterprise and government and these can be changed by changing those policies. Indeed, virtually all of the policies of government and business have effects upon health, and they are likely to have a top down effect via the brain on all the physiological systems involved in stress and adaptation.5 For example, programs that promote physical activity are likely to benefit brain function (see above), just as programs such as the Experience Corps produce benefits to the elderly volunteers in both physical and mental health.36 Likewise, studies of the efficacy of programs for children, such as the Perry School Project,b would benefit from assessment of cognitive function and brain health. Therefore, monitoring how the brain is affected by such policies is another important future direction of neuroimaging research because animal models can only give clues, but the study of the adaptability of the human brain is the ultimate goal!
References:
- Gunnar MR, Herrera A, Hostinar CE. Stress and early brain development. In: Tremblay RE, Barr RG, Peters RDeV, Boivin M, eds. Encyclopedia on Early Childhood Development [online]. Montreal, Quebec: Centre of Excellence for Early Childhood Development; 2009:1-8 Available at: http://www.child-encyclopedia.com/documents/Gunnar-Herrera-HostinarANGxp.pdf. Accessed January 8, 2010.
- Heim C. Childhood trauma and adult responsiveness. In: Tremblay RE, Barr RG, Peters RDeV, Boivin M, eds. Encyclopedia on Early Childhood Development [online]. Montreal, Quebec: Centre of Excellence for Early Childhood Development; 2009:1-7 Available at: http://www.child-encyclopedia.com/documents/HeimANGxp.pdf. Accessed January 8, 2010.
- Shonkoff JP, Boyce WT, McEwen BS. Neuroscience, molecular biology, and the childhood roots of health disparities. JAMA: Journal of the American Medical Association 2009;301(21):2252-2259.
- McEwen BS. Protective and damaging effects of stress mediators. New England Journal of Medicine 1998;338(3):171-179.
- McEwen BS. Physiology and neurobiology of stress and adaptation: Central role of the brain. Physiology Reviews 2007;87(3):873-904.
- McEwen BS. Protective and damaging effects of stress mediators: Central role of the brain. Dialogues in Clinical Neurosciences 2006;8(4):367-381.
- Calabrese EJ. Neuroscience and hormesis: Overview and general findings. Critical Review in Toxicology 2008;38(4):249-252.
- McEwen BS, Wingfield JC. The concept of allostasis in biology and biomedicine. Hormones and Behavior 2003;43(1):2-15.
- Karlamangla AS, Singer BH, Seeman TE. Reduction in allostatic load in older adults is associated with lower all-cause mortality risk: MacArthur Studies of Successful Aging. Psychosomatic Medicine 2006;68(3):500-507.
- Seeman TE, Crimmins E, Huang MH, Singer B, Bucur A, Gruenewald T, Berkman LF, Reuben DB. Cumulative biological risk and socio-economic differences in mortality: MacArthur studies of successful aging. Social Science & Medicine 2004;58(10):1985-1997.
- Seeman TE, McEwen BS, Rowe JW, Singer BH. Allostatic load as a marker of cumulative biological risk: MacArthur studies of successful aging. Proceedings of the National Academy of Sciences of the United States of America 2001;98(8):4770-4775.
- Seeman TE, Singer BH, Ryff CD, Dienberg G, Levy-Storms L. Social relationships, gender, and allostatic load across two age cohorts. Psychosomatic Medicine 2002;64(3):395-406.
- McEwen BS. The physiology and neurobiology of stress and adaptation: Central role of the brain. Physiological Reviews 2007;87(3):873-904.
- Gianaros PJ, Jennings JR, Sheu LK, Greer PJ, Kuller LH, Matthews KA. Prospective reports of chronic life stress predict decreased grey matter volume in the hippocampus. Neuroimage 2007;35(2):795-803.
- Gold SM, Dziobek I, Sweat V, Tirsi A, Rogers K, Bruehl H, Tsui W, Richardson S, Javier E, Convit A. Hippocampal damage and memory impairments as possible early brain complications of type 2 diabetes. Diabetologia 50(4):711-719.
- Sheline YI. Neuroimaging studies of mood disorder effects on the brain. Biological Psychiatry 2003;54(3):338-352.
- Cho K. Chronic ‘jet lag’ produces temporal lobe atrophy and spatial cognitive deficits. Nature Neuroscience2001;4(6):567-568.
- Marsland AL, Gianaros PJ, Abramowitch SM, Manuck SB, Hariri AR. Interleukin-6 covaries inversely with hippocampal grey matter volume in middle-aged adults. Biological Psychiatry 2008;64(4):484-490.
- Pruessner JC, Balwin MW, Dedovic K, Renwick R, Mahani NK, Lord C, Meaney M, Lupien S. Self-esteem, locus of control, hippocampal volume, and cortisol regulation in young and old adulthood. Neuroimage 2005;28(4):815-826.
- Yamada K, Nabeshima T. Stress-induced behavioral responses and multiple opioid systems in the brain. Behavioural Brain Research 1995;67(2):133-145.
- Colcombe SJ, Kramer AF, McAuley E, Erickson KI, Scalf P. Neurocognitive aging and cardiovascular fitness: Recent findings and future directions. Journal of Molcular Neuroscience 2004;24(1):9-14.
- Kramer AF, Hahn S, Cohen NJ, Banish MT, McAuley E, Harrison CR, Chason J, Vakil E, Bardell L, Boileau RA, Colcombe A. Ageing, fitness and neurocognitive function. Nature 1999;400(6743):418-419.
- Abe H, Keen KL, Terasawa E. Rapid action of estrogens on intracellular calcium oscillations in primate luteinizing hormone-releasing hormone-1 neurons. Endocrinology 2008;149(3):1155-1162.
- Drevets WC, Price JL, Simpson JR Jr, Todd RD, Reich T, Vannier M, Raichle ME. Subgenual prefrontal cortex abnormalities in mood disorders. Nature 386(6627):824-827.
- Gianaros PJ, Horenstein JA, Cohen S, Matthews KA, Brown SM, Flory JD, Critchley HD, Manuck SB, Hariri AR. Perigenual anterior cingulate morphology covaries with perceived social standing. Social Cognitive and Affective Neuroscience 2007;2(3):161-173.
- Gianaros PJ, Sheu LK, Matthews KA, Jennings JR, Manuck SB, Hariri AR. Individual differences in stressor-evoked blood pressure reactivity vary with activation, volume, and functional connectivity of the amygdala. Journal of Neuroscience 2008;28(4):990-999.
- Olsson A, Phelps EA. Social learning of fear. Nature Neuroscience 2007;10(9):1095-1102.
- Gianaros PJ, Jennings JR, Sheu LK, Derbyshire SW, Matthews KA. Heightened functional neural activation to psychological stress covaries with exaggerated blood pressure. Hypertension 2007;49(1):134-140.
- Weil ZM, Norman GJ, Barker JM. Social isolation potentiates cell death and inflammatory responses after global ischemia. Molecular Psychiatry 2008;13(10):913-915.
- Schwartz JM, Stoessel PW, Baxter LR Jr, Martin KM, Phelps ME. Systematic changes in cerebral glucose metabolic rate after successful behavior modification treatment of obsessive-compulsive disorder. Archives of General Psychiatry 1996;53(2):109-113.
- de Lange FP, Koers A, Kalkman JS, Bleijenberg G, Hagoort P, van der Meer JW, Toni I. Increase in prefrontal cortical volume following cognitive behavioural therapy in patients with chronic fatigue syndrome. Brain 2008;131(8):2172-2180.
- Balasubramanian B, Portillo W, Reyna A, Chen JZ, Moore AN, Dash PK, Mani SK. Nonclassical mechanisms of progesterone action in the brain: II. Role of calmodulin-dependent protein kinase II in progesterone-mediated signaling in the hypothalamus of female rats. Endocrinology 2008;149(11):5518-5526.
- Lupien SJ, McEwen BS, Gunnar MR, Heim C. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nature Reviews Neuroscience 2009;10(6):434-445.
- Evans GW, Schamberg MA. Childhood poverty, chronic stress, and adult working memory. Proceedings of the National Academy of Sciences of the United States of America 2009;106(16):6545-6549.
- Farah MJ, Shera DM, Savage JH, Betancourt L, Giannetta JM, Brodsky NL, Malmud EK, Hurt H. Childhood poverty: Specific associations with neurocognitive development. Brain Research 2006; 1110(1):166-174.
- Fried LP, Carlson MC, Freedman M, Frick KD, Glass TA, Hill J, McGill S, Rebok GW, Seeman T, Tielsch J, Wasik BA, Zeger S. A social model for health promotion for an aging population: Initial evidence on the experience corps model. Journal of Urban Health 2004;81(1):64-78.
Notes:
a See also the Nurse Family Partnership website available at: http://www.nursefamilypartnership.org. Accessed December 17, 2009.
b See also: HighScope Educational Research Foundation. HighScope Perry Preschool Study: Lifetime effects: The HighScope Perry Preschool Study through age 40; 2005. Available at: http://www.highscope.org/Content.asp?ContentId=219. Accessed December 17, 2009.
How to cite this article:
McEwen BS. The Brain: The Central Organ of Stress and Adaptation Across the Lifecourse. In: Tremblay RE, Boivin M, Peters RDeV, eds. Encyclopedia on Early Childhood Development [online]. https://www.child-encyclopedia.com/brain/according-experts/brain-central-organ-stress-and-adaptation-across-lifecourse. Published: July 2010. Accessed April 16, 2024.
Text copied to the clipboard ✓