Neurological Adverse Events After Vaccination
Barbara Law, MD, (FRCPC)
Public Health Agency of Canada, Canada
Introduction
The first five years of a child’s life are filled with developmental milestones. It is also a time for health promotion including scheduled immunizations to prevent up to 14 infections (Table 1) that were once common causes of childhood injury and death. Finally it may be a time when health problems first appear, as a result of genetic inheritance or injuries before, during or after birth.
For parents it is an exceptionally busy time full of wonder, joy, fatigue and fear as they strive to be the best possible caretakers and make good choices for their children to ensure health and avoid injury. Given the safety and benefit of vaccines, immunization is an excellent choice.1 Still there are understandable concerns that immunizing an apparently healthy child may cause harm. Decisions should be based on the best available evidence.
Subject
This article summarizes the type and frequency of neurologic events proven to be caused by vaccines. Fortunately the list is short and easily summarized (Table 2). It also covers how vaccines are assessed before and after a product is marketed and some of the special challenges related to monitoring safety during early childhood.
Problems
It was once thought that all aspects of safety could be learned prior to product approval by regulatory authorities. The thalidomide disaster midway through the 20th century changed that. Thalidomide was a product given to pregnant women to prevent morning sickness. Pre-licensure studies failed to identify a significant side effect – notably limb shortening that occurred during intrauterine development of infants whose mothers took the drug. This led to a global declaration by the World Health Assembly in 1963 and the modern era pharmacovigilance was born.2
Vaccine pharmacovigilance is defined as the science and activities relating to the detection, assessment, understanding, prevention and communication of adverse events following immunization, or of any other vaccine-or immunization-related issues.3
Research Context
Currently, vaccines are among the safest of marketed products thanks to stringent regulatory standards and continuous product testing and monitoring before and after licensure.4 Regulators such as Health Canada only approve a vaccine if it is shown that the product works as intended (“efficacious”) and is safe. From the time of vaccine discovery throughout the life of a product, vaccine manufacturers must follow a set of international quality management standards (“good practices” related to laboratory, clinical study and manufacturing processes) to ensure the validity of studies used to support their claims for product safety and efficacy as well as the ongoing consistency and quality of the manufacturing process.
Much is learned about the safety profile of the vaccine during these studies including the frequency and severity of common side effects (affecting more than 1% of vaccine recipients) like injection site reactions and fever.5 The presence or absence of rare side effects (affecting from 1 in 1,000 to 1 in 10,000 vaccine recipients) may also be confirmed.
Still, it is impossible to know everything about a vaccine before it is used in large populations. Very rare events (less than once for every 10,000 doses) can’t be identified because of study size limitations. Seldom are there data on the safety profile in sub-populations such as premature infants or those with chronic disease. Thus additional studies as well as ongoing monitoring are needed to answer remaining questions concerning a vaccine product safety profile. These may require special techniques since once a vaccine is proven effective as it may be unethical to use unimmunized controls.6-9
Key Research Questions
There are three key questions to ask regarding vaccine administration and a subsequent adverse effect:10
1. Can it? The answer to this comes from well designed studies that seek to confirm or reject the hypothesis that a vaccine can cause an effect (“causal association”). If there is an association, even if rare, one can usually prove it, provided a large enough group is studied using appropriate methods. Live vaccines have the potential to cause the same adverse effects as the wild target viruses but, because they are attenuated, do so at much reduced frequency.
2. Will it? This is the question most relevant to parents because it addresses the likelihood that an event will follow immunization. It would be good to have an absolute “yes or no” answer to the question, but that is no more possible than predicting any future event. Having the answer to Can it? is an essential part of considering Will it?, especially if an answer for How often can it? is also known.
3. Did it? This is the hardest question to answer and the one that is most poorly understood. What is actually being asked is:If the vaccine hadn’t been given, would the event still have happened?
Reporting systems for adverse events following immunization (AEFI) are intended to identify possible signals of a vaccine safety problem.5 A signal could be increased frequency and/or severity of a known side effect or a previously unrecognized adverse event. The signal is a flag of a possible concern but it is still necessary to address the Can it? question with appropriately-designed studies. Depending on the seriousness of the concern, regulators may stop or limit use of a particular vaccine lot or product until the question can be answered.
Some AEFI reporting systems, such as VAERS (Vaccine Adverse Event Reporting System) in the U.S. are publicly accessible.11 To interpret VAERS and similar data it is essential to understand what an AEFI is and what it is not. In the most general sense an AEFI is “Any untoward medical occurrence which follows immunization and which does not necessarily have a causal relationship with the usage of the vaccine. The AE may be any unfavourable or unintended sign, abnormal laboratory finding, symptom or disease.”3 The AEFI reporter need only suspect that the vaccine could have caused the event to submit a report. However, a report is not proof that the vaccine caused the event. Rather there are five different possibilities that could have resulted in the adverse events (AE) of which four are related to vaccine and/or immunization and one is not:3
i. Vaccine product-related reaction: An AEFI that is caused or precipitated by a vaccine due to one or more of the inherent properties of the vaccine product.
Example: febrile seizure occurring six to 14 days after measles-mumps-rubella (MMR) vaccine.8
ii. Vaccine quality defect-related reaction: An AEFI that is caused or precipitated by a vaccine, due to one or more quality defects of the vaccine product including its administration device as provided by the manufacturer.
Example: shortly after licensure of the Salk inactivated polio vaccine in 1955, some lots prepared by one manufacturer (Cutter laboratory) contained incompletely inactivated poliovirus, resulting in paralytic polio in several recipients. The Cutter vaccine was taken off the market but vaccine from other manufacturers’ was safe for use and epidemic polio became a distant memory.12 Additionally the incident led to more effective regulatory control of vaccines.
iii. Immunization error-related reaction: An AEFI that is caused by inappropriate vaccine handling, prescribing or administration and thus by its nature is preventable.
Example: the live attenuated measles vaccine can cause fatal encephalitis if given to individuals whose immune system is weak or not working due to genetic or acquired disease or immune suppression therapy.13 Live vaccines like MMR are contraindicated in such individuals. Failure to adhere to such contraindications is an example of this type of AEFI.
iv. Immunization anxiety-related reaction: An AEFI arising from anxiety about the immunization.
Example: fainting episode shortly after immunization, sometimes, accompanied by jerking of the limbs which can be mistaken for a seizure, by onlookers. This is uncommon in preschool-aged children.14
v. Coincidental event: An AEFI that is caused by something other than the vaccine product, immunization error or immunization anxiety.”
Example 1: underlying disease – as pointed out above, the first five years of life is a time when genetic diseases or unrecognized injuries acquired before, during or after birth may first become apparent. When the first onset follows soon after immunization it is an AEFI – associated in time but not caused by the vaccine – it would have happened whether the vaccine was given or not.
Example 2: early childhood is also a time of frequent infections which are usually fairly benign causing colds, ear infections, stomach upset or diarrhoea but which sometimes cause more serious complications including meningitis or encephalitis. Side effects can also be due to the drugs used to cure or relieve symptoms of such infections.
Given the fact that “coincidental events” may present as an AEFI, another important research question is: What is the incidence of neurological adverse effects in child populations in the absence of vaccination (commonly referred to as “background rate”), including any variations that depend on geographic location or seasonal variation as well as subgroups with increased risk.15 Knowing the expected rates of events helps in monitoring vaccine safety where the goal is to detect any unexpected increased frequency when a new vaccine is introduced.
Recent Research Results
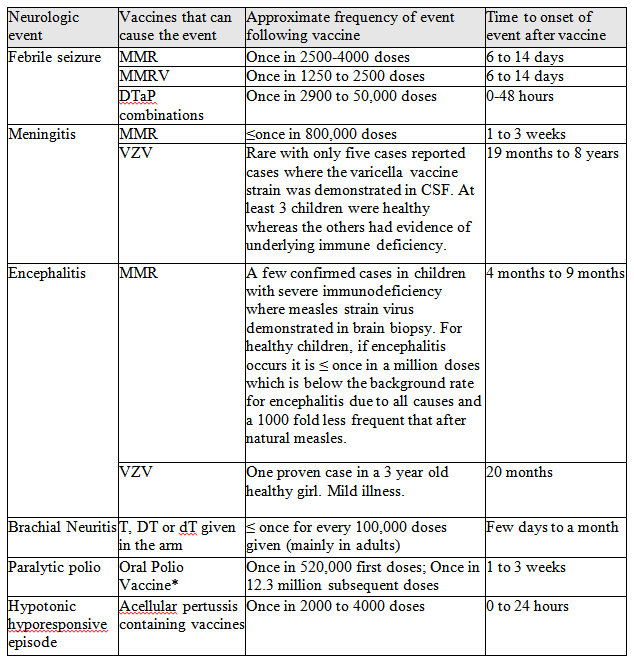
Table 2 summarizes the proven links between vaccine and neurologic adverse events as reviewed by the Institute of Medicine (IOM).10,13,16-20 While only MMR, MMR-varicella and diphtheria-tetanus-acellular pertussis (DTaP) vaccines are listed as causes of febrile seizure, it is likely that any vaccine that causes fever could also induce a febrile seizure, especially in those with a personal or family history of such events. Note that the frequency of meningitis and encephalitis is much lower after the live viral vaccines than after infection with the wild viruses.
Research Gaps
In their exhaustive recent review of the evidence linking vaccines to serious adverse events13 the Institute of Medicine concluded that “… some issues simply cannot be resolved with currently available epidemiologic data excellent as some of the collections and studies are. Particularly for rare events, we look to the day when electronic medical records truly are universal and when society reaches a broad-based consensus about how these records may be used to detect very rare adverse events from vaccines as well as other drugs and medical interventions.” The IOM report also noted that expert guidelines are needed regarding AEFI investigation in order to rule out coincidental events. Finally, a better understanding is needed of genetic and other factors that may increase the risk of suffering an adverse reaction.21
Conclusions
Vaccines are among the safest and most effective products used today thanks to ongoing efforts to standardize research and manufacturing practices before and after licensure as well as constant monitoring for signal detection and subsequent investigations to test whether a vaccine truly causes a given adverse event.
Febrile seizures are the only neurologic reactions that occur with any frequency following infant and childhood immunizations, and even these are rare, occurring less than once for every thousand doses of vaccine given. While frightening to witness, they are benign and don’t cause lasting harm nor do they go on to become epilepsy. Hypotonic hyporesponsive episodes (HHE) occur with a similar frequency as febrile seizures, predominantly in infants following immunization with pertussis containing combination vaccines. HHE episodes, in which an infant becomes pale, floppy like a rag doll and less responsive, may represent a type of fainting spell. They cause no lasting effect and infants having had one episode are at no increased risk for another.
Implications for Parents, Services and Policy
When parents choose to immunize their children according to recommended schedules, they are opting for effective and safe measures to maintain health by preventing disease. While no vaccine is 100% safe, the benefit greatly outweighs any potential harm. Still research has clearly linked certain events, like febrile seizures, to vaccines. It is essential that vaccine and other health care providers give accurate information regarding common and rare side effects along with advice on what to do should an adverse event occur. Trustworthy information can also be found at several websites.22-25 It is equally important that all those concerned with immunization of young children remain vigilant to the possibility of AEFIs and that they not only report them but also investigate appropriately to ensure that vaccines and immunization programs remain as safe as they can be and also that coincidental events are properly diagnosed and treated.
TABLE 1. Diseases targeted by vaccines given during the first 5 years of life
| Approximate timing to start immunization* | Diseases prevented |
| Birth | Hepatitis B |
| First 6 months |
Rotavirus gastroenteritis |
| 6 to 23 months | Influenza |
| 12 to 15 months | Measles Mumps Rubella Chickenpoz |
*Immunization schedules vary from province to province and from one country to another. For specific information in a given area it is best to ask your healthcare provider or local public health.
TABLE 2. Synthesized evidence regarding neurologic events proven to be caused by vaccine(s). The data summarized in the table are meant to give a general overview of what is known based on published data as reviewed by the US Institute of Medicine.10,13,16-19 The listed vaccines are those currently available in North America unless otherwise specified.
References
- Public Health Agency of Canada. A Parent’s Guide to Immunization. Available at: http://www.phac-aspc.gc.ca/im/iyc-vve/index-eng.php. Accessed April 8, 2013.
- World Health Organization. The safety of medicines in public health programmes: Pharmacovigilance an essential tool. 2006.
- CIOMS/WHO Working Group on Vaccine Pharmacovigilance. Definition and Application of Terms for Vaccine Pharmacovigilance. Council for International Organizations of Medical Sciences (CIOMS) 2012.
- Dellepiane N, Griffiths E, Milstien JB. New challenges in assuring vaccine quality. Bull WHO 2000; 78:155-162.
- National Advisory Committee on Immunization. Part 2. Vaccine safety and adverse events following immunization. Canadian Immunization Guide. 7th ed. Ottawa, Ontario: Health Canada; 2006.
- Farrington CP. Control without separate controls: evaluation of vaccine safety using case-only methods. Vaccine 2004; 22:2064-70.
- Chen RT, DeStefano F, Davis RL et al. The Vaccine Safety Datalink : immunization research in health maintenance organizations in the USA. Bull WHO 2000; 78:186-94.
- Andrews NJ. Statistical assessment of the association between vaccination and rare adverse events post-licensure. Vaccine 2002; 20:S49-S53.
- HviidA. Postlicensure epidemiology of childhood vaccination: the Danish experience.Expert Rev Vaccines 2006; 5:641-9.
- Stratton KR, Howe CJ, Johnston RB, eds; Vaccine Safety Committee, Division of Health Promotion and Disease Prevention, Institute of Medicine. Adverse Events Associated with Childhood Vaccines. Evidence Bearing on Causality. Washington, DC: National Academy Press; 1994.
- Varricchio F, Iskander J, DeStefano F et al. Understanding vaccine safety information from the Vaccine Adverse Event Reporting System. Pediatr Infect Dis J 2004; 23:287-94.
- Offfit PA. The Cutter Incident: How America’s first polio vaccine led to the growing vaccine crisis. Yale University Press 2005.
- Stratton K, Ford A, Rusch E, Wright Clayton E, eds; Committee to Review Adverse Effects of Vaccine, Board on Population Health and Public Health Practice, Institute of Medicine. Adverse Effects of Vaccine: Evidence and Causality. Washington, DC: National Academies Press; 2011
- Braun MM, Patriarca PA, Ellenberg SS. Syncope alter immunization. Arch Pediatr Adolesc Med 1997; 151:255-9.
- Black S, Eskola J, Siegrist CA et al. Importance of background rates of disease in assessment of vaccine safety during mass immunixsation with pandemic H1N1 influenza vaccines. Lancet 2009; 374:2115-22.
- Howson CP, Howe CJ, Fineberg HV, eds; Committee to Review the Adverse Consequences of Pertussis and Rubella Vaccines, Institute of Medicine. Adverse effects of pertussis and rubella vaccines. Washington, DC: National Academy Press; 1991.
- Stratton K, Almario DA, McCormick MC, eds; Immunization Safety Review Committee, Board on Health Promotion and Disease Prevention, Institute of Medicine. Immunization Safety Review. Hepatitis B Vaccine and Demyelinating Neurological Disorders. Washington, DC: National Academies Press, 2002.
- Stratton K, Almario DA, Wizemann T, McCormick MC, eds; Immunization Safety Review Committee, Board on Health Promotion and Disease Prevention, Institute of Medicine. Immunization Safety Review. Influenza Vaccines and Neurological Complications. Washington, DC: National Academies Press, 2004.
- Stratton K, Gable A, Shetty P, McCormick M, eds. Immunization Safety Review: Measles-Mumps-Rubella Vaccines and Autism. Washington, DC: Institute of Medicine, National Academies Press; 2001.
- World Health Organization. The Global Advisory Committee on Vaccine Safety (GACVS) – Topics covered in committee meetings. Available at: http://www.who.int/vaccine_safety/topics/en. Accessed April 8, 2013.
- Poland GA. Vaccidents and adversomics. Vaccine 2010; 28:6549-50.
- Canadian Paediatric Society (CPS) / Caring for Kids. Immunization. Available at: http://www.caringforkids.cps.ca/handouts/immunization-index. Accessed April 8, 2013.
- Immunize Canada. Available at: http://immunize.ca. Accessed April 8, 2013.
- Public Health Agency of Canada. Available at: http://www.phac-aspc.gc.ca/im/index-eng.php. Accessed April 8, 2013.
- World Health Organization Vaccine Safety Net. Available at: http://www.who.int/immunization_safety/safety_quality/approved_vaccine_safety_websites/en/index.html. Accessed April 8, 2013.
How to cite this article:
Law B. Neurological Adverse Events After Vaccination. In: Tremblay RE, Boivin M, Peters RDeV, eds. Scheifele DW, topic ed. Encyclopedia on Early Childhood Development [online]. https://www.child-encyclopedia.com/immunization/according-experts/neurological-adverse-events-after-vaccination. Published: April 2013. Accessed April 22, 2024.
Text copied to the clipboard ✓