Childhood Immunization and Brain Health
David W. Scheifele, MD
University of British Columbia, Child & Family Research Institute, Canada
Introduction and Subject
The benefits of routine childhood immunizations can be described from many perspectives, such as deaths avoided, health care costs saved and suffering spared, each of which is important. The World Health Organization (WHO) and UNICEF estimate that basic childhood immunizations (Table 1) are given to over 100 million children, save over 2.5 million lives annually and could save 2 million more lives if fully implemented.1 Childhood immunizations either save money (e.g., measles vaccine) or represent good value (e.g., meningitis vaccines). Most of the infections targeted by vaccines can cause neurological injuries (Table 2) so disease control avoids those complications and preserves brain health. The objective of this paper is to describe briefly the most common neurological conditions that are avoidable with routine childhood immunizations, recognizing that not all of the vaccines described are used in all countries and that disease risks vary with geography and population health (e.g., prevalence of HIV infections). The information is meant to assist health care providers to understand and explain the principal advantages of vaccination.
Recent Research Results
1. Contagious Infections of Childhood
The highly contagious childhood infections associated with fever and rash (measles, rubella, chickenpox) or parotitis (mumps) are infrequent causes of neurological complications but because they affect nearly every child, the cumulative numbers of neurological injuries become significant.
1a. Measles
Measles infection causes particularly high fevers that trigger febrile convulsions in about 1/25-30 cases. While seizures are frightening for parents, most simple febrile seizures are without consequences. However, some atypical febrile seizures can be prolonged (status epilepticus) and without prompt medical attention and effective airway care can pose a risk of hypoxic brain injury.
Measles is complicated by acute encephalitis in about 1/1,000 cases. In a country with a birth rate of 100,000 children per year, that means ~100 children will suffer from measles encephalitis annually and cumulatively ~1,500 children will be affected by age 15 years. Measles encephalitis is typically severe, with a high rate of subsequent brain injury, developmental delay and ongoing seizures among survivors.2 A post-infectious encephalomyelitis also occurs, with autoimmunity to myelin basic protein (MBP).3
Measles infection can also initiate slowly progressive encephalitis. In immunocompromised children, measles inclusion body encephalitis develops after about six months. A slower infection, known as subacutesclerosing panencephalitis (SSPE), affects previously healthy children seven to 10 years after their measles illness and progresses to a vegetative state and death within two to three years of becoming evident.4 It is fortunately rare, affecting about one per million children.
With measles vaccination, both acute and chronic forms of encephalitis are avoided and the risk of febrile convulsions is markedly reduced. Vaccination causes fever in ~5% of first-time vaccinees and is occasionally high enough to trigger seizures in prone individuals. The estimated rate of febrile seizures after measles-mumps-rubella (MMR) vaccination in the USA was about 1/3,000, a rate 100-fold lower than after measles infection.5
1b. Mumps
Mumps infection is ordinarily benign but results in central nervous system (CNS) infections with surprising frequency. As many as 1/10 cases will have meningeal inflammation, potentially presenting with severe headache and neck stiffness, with cerebrospinal fluid (CSF) pleocytosis. This is typically self-limiting, resolving within a week. About 10% of CNS cases (1/1,000 children) will be more severe, presenting with encephalitis and/or nerve deafness. In the absence of vaccination programs, mumps is a significant contributor to acquired deafness. Mumps encephalitis is typically severe and prolonged and can result in permanent brain injury with hydrocephalus, developmental delay and ongoing seizures.6
Mumps vaccines are well tolerated but occasionally cause mild aseptic meningitis. Rates vary by vaccine virus.7 Outcomes are generally benign, with symptoms resolving within a few days.
1c. Rubella
Acute rubella infection is generally benign for healthy children, posing a low risk of acute encephalitis. However, children with severe immunodeficiency can develop a chronic, progressive form of rubella encephalitis. Of greatest concern, however, is the occurrence of congenital rubella syndrome (CRS) following infection in utero.8 This typically results in severe neurological damage, including microcephaly, cortical malformations, blindness, deafness, hydrocephalus and persistent seizures. The WHO recommends administration of combined rubella and measles vaccines to young children, whenever feasible, to prevent CRS. Rubella immunization of adolescent girls and susceptible women contemplating pregnancy are additional control strategies. Universal vaccination of young children and selective vaccination of susceptible women quickly resulted in the elimination of CRS cases in the U.S.9 and the Americas.10 Modern rubella vaccines have an excellent safety record when given to infants and young children.
1d. Varicella (Chickenpox)
About 1% of children with chickenpox suffer a significant complication requiring medical attention, with CNS complications prominent among them.11,12 The latter can include febrile seizures, aseptic meningitis, cerebellitis, encephalitis and transverse myelitis. A distinctive CNS complication is cerebellitis, a focal encephalitis that presents with dizziness and ataxia, but from which patients generally recover well. 13 Reye’s syndrome, a life-threatening disorder which almost always follows a viral illness such as chickenpox or a cold, is even more of a risk ASA exposure. Hemiparesis or hemiplegia may occur abruptly weeks or months after the rash illness as a result of stroke secondary to cerebral arteritis.14 Varicella causes persistent infection of paraspinal ganglia so that later in life about 25% of infected individuals will experience reactivation of the latent virus, causing the vesicular, dermatomal rash of zoster. Zoster complications include neuralgia, which can be excruciating and persist for months, meningeal inflammation (aseptic meningitis) and, less commonly, transverse myelitis.
Varicella vaccination prevents neurologic complications and, later, zoster. The attenuated vaccine virus can also persist in ganglia but the rate of post-vaccination zoster is lower than with wild infection and the illness is much milder, with minimal neuralgia.
2. Neurotropic viruses
This category includes poliomyelitis, rabies, Japanese encephalitis and tick-borne encephalitis, each of which is neurotropic and vaccine preventable.
2a. Poliomyelitis
Polioviruses replicate in and spread from the gastrointestinal tract, making them highly contagious. Most infected persons are asymptomatic or mildly ill with fever but about 1% experience multi-focal infection of spinal motor neurons, with abrupt onset of weakness or paralysis. Brain stem involvement (bulbar polio) compromises respiration, necessitating assisted ventilation. With limited loss of motor neurons, recovery of function is possible. With greater losses, paralysis or weakness is permanent, with further risk of joint contractures and limb deformity in the absence of good physical therapy. The disease is epidemic at times but is otherwise endemic, causing sporadic cases.
Extensive global vaccination programs have eliminated circulation of wild polio viruses from most of the world. Mass vaccination programs aimed at eliminating the disease altogether have been highly successful: in 2011 disease activity was confined to just four remaining endemic areas (Nigeria, India, Pakistan and Afghanistan), where eradication efforts continue.15
3. Invasive Bacterial Infections
During first encounters with certain encapsulated bacteria, young children are at increased risk of bloodstream invasion, with spread of the pathogen to the meninges. Without prompt recognition and antibiotic treatment, meningitis can result in permanent brain injury. The leading pathogens have been the targets of vaccine development and subsequent disease control programs.
3a. Haemophilus influenzae type b
Once the leading cause of invasive bacterial infection in children, H. influenzae b (Hib) was estimated to affect 1/200 American children by 5 years of age.16 About half of Hib infections involved purulent meningitis, complications of which included deafness (unilateral or bilateral, in 20% of survivors) and neurological sequelae (in 15%) that included developmental delay, hydrocephalus, seizure disorder, blindness, and motor impairments.17 Injury resulted primarily from damage to cerebral vessels entrapped in the inflammatory process. Direct invasion of brain tissue was rare without antecedent focal hypoxic damage (cerebritis) but this mechanism could lead to cortical abscess formation. Hib meningitis was the leading cause of acquired deafness among children and the leading post-natal cause of developmental delay.
Where routine use of Hib conjugate vaccines has been well implemented, disease rates have fallen sharply, reducing case numbers by 99% or more.18 In 2009, 82% of WHO-registered countries supplied Hib vaccines, affording protection to about 45% of young children globally.19 Hib vaccines have an excellent safety record.
3b. Invasive Pneumococcal Infections
Second to Hib in causing invasive infections in children, Streptococcus pneumoniae bacteria less often infect the meninges: about 15% of cases involve purulent meningitis.20 The outcome of meningitis cases is similar after either infection, with deafness occurring in 30% of pneumococcal cases in one series21 and cortical damage in 15%.
The cumulative risk of pneumococcal invasive disease (IPD) for children <5 years of age in Canada22 was estimated at one in 330 children with the risk of meningitis being about one-tenth of that. Estimated IPD incidence rates in the developing world are much higher,23 even allowing for under-diagnosis, with meningitis accounting for a larger proportion of recognized cases.
The availability of multivalent pneumococcal conjugate vaccines has provided a means to substantially reduce the frequency of invasive pneumococcal infections, including meningitis.23 These vaccines have not been associated with neurologic adverse events apart from occasional febrile seizures.
3c. Meningococcal Infections
Invasive meningococcal infections are feared for their abrupt onset and severity but are fortunately uncommon in developed countries, with incidence rates <1 per 100,000 population per year. Nevertheless children <2 years of age and adolescents have the highest incidence rates. About 50% of cases involve meningitis,24 with the same range of potential complications as with Hib and pneumococcal meningitis, including deafness in about 10% of survivors.25,26
Immunization programs with serogroup C and/or tetravalent (ACYW135) conjugate vaccines have been effective, reducing rates of invasive and meningeal infections.27 However, serogroup B meningococci are not yet vaccine preventable and account for a substantial proportion of remaining meningitis cases.
3d. Tuberculous meningitis
Tuberculous meningitis occurs as a complication of primary pulmonary tuberculosis, typically among young children. Failure to confine infection to the lungs results in seeding of the meninges, which initiates an inflammatory response particularly around the brain stem. Injury to cerebral vessels leads toischemic brain stem and cortical injury. Outcome of modern multi-drug treatment is often poor, especially when children present in coma. Sequelae are frequent among survivors. Primary prevention with BCG vaccine is a better option. Currently available BCG vaccines are about 50% effective in preventing pulmonary infection but are ~64% effective in reducing progression to meningitis.28,29
4. Other Infections Occasionally Causing Brain Injury
In this category are preventable infections of childhood that can indirectly result in neurological injury, such as pertussis, influenza and tetanus.
4a. Pertussis
Seizures and encephalopathy are uncommon complications of pertussis, reported in about 2.5 and 0.5% of hospitalized cases, respectively.30 Encephalopathy is believed to result from hypoxic injury suffered during lengthy bouts of coughing or apnea. Petechial hemorrhages may also occur in brain tissue during heavy, prolonged coughing, contributing to seizures and encephalopathy. About half of survivors of encephalopathy have permanent brain damage.
Immunization with whole cell or acellular pertussis vaccines has markedly reduced neurological injuries from pertussis. Vaccines are about 80% effective in preventing infection for periods of 5-10 years and reduce the risk of hospitalization and death among cases that occur despite vaccination.
Conclusion
Most of the infections targeted by childhood vaccines have the potential to cause neurologic complications and subsequent disability. Taken together, the burden for parents and society in caring for affected children is substantial. Fortunately, childhood vaccinations are effective in preventing infections and their neurologic complications. When well used, vaccines contribute significantly to the preservation of brain health in children.
TABLE 1
WHO Recommended Vaccines for All Children (11/2012)
| Antigen | Comment | ||
| BCG tuberculosis | For infants in high-risk countries or households, except those with HIV infection | ||
| Hepatitis B | Starting as soon after birth as possible | ||
| Polio | 3 infant doses of OPV recommended in polio-endemic countries; IPV used in polio-free countries | ||
| DPT | 3-dose series in infancy, whole cell or acellular pertussis vaccine, with booster dose at 1-6 years of age | ||
| H. influenzae b | 3-dose series in infancy, combination vaccine preferred where available or with concurrent DTP | ||
| Pneumococcal conjugate | 3- dose series in infancy, 2 alternative schedules | ||
| Rotavirus | 2- or 3- dose series in early infancy, depending upon product | ||
| Measles | 1st dose given at 9 or 12 months; 2nd dose at 15-18 months | ||
| Rubella | 1 dose minimum; ideally given in combined vaccine with measles, i.e. Measles-Rubella (MR) or Measles-Mumps-Rubella (MMR) | ||
| HPV | Females before onset of sexual activity; 3-dose series | ||
Refer to http://www.who.int/immunization/documents/positionpapers/ for most recent schedule and details for use of these and other vaccines recommended for certain regions, some high-risk populations and immunization programs with certain characteristics.
TABLE 2
Infection-related Neurological Injuries Avoidable with Childhood Immunization
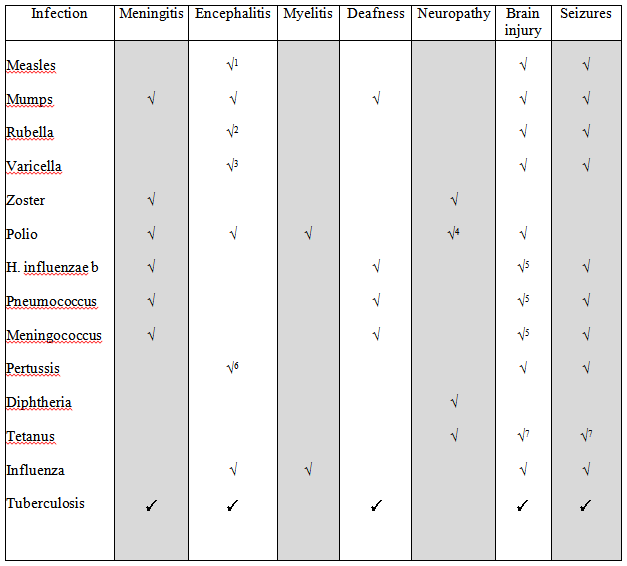
1 Measles – includes subacute sclerosing panencephalitis in previously healthy children
2 Rubella – includes chronic encephalitis in immunocompromised children
3 Varicella – includes acute cerebellitis and vascular stroke
4 Polio – neuropathy as post-polio syndrome
5 Brain injury with meningitis mediated mainly through cerebrovascular injury; also subsequent hydrocephalus
6 Pertussis encephalopathy is hypoxia-related
7 Tetanus brain injury is hypoxia-related
References
- WHO, UNICEF, World Bank. State of the world’s vaccines and immunization, 3rd edition, Geneva, World Health Organization, 2009.
- Aarli J A. Nervous complications of measles: clinical manifestations and prognosis. Eur Neurol 1974; 12:79-93.
- Johnson R T, Griffin D E, Hirsch R L et al. Measles encephalomyelitis – clinical and immunologic studies. N Engl J Med 1984; 310:137-41.
- Sever J. Persistent measles infection of the central nervous system: subacute sclerosing panencephalitis. Rev Infect Dis 1983; 5:467-73.
- Barlow W E, Davis R L, Glasser J W et al. The risk of seizures after receipt of whole-cell pertussis or measles, mumps, and rubella vaccine. N Engl J Med 2001; 345:656-61.
- MacDonald J C, Moore D L, Quennec P. Clinical and epidemiological features of mumps meningoencephalitis and possible vaccine-related disease. Pediatr Infect Dis J 1989; 8:751-5.
- Bonnet M C, Dutta A, Weinberger C, Plotkin S A. Mumps vaccine virus strains and aseptic meningitis. Vaccine 2006; 24:7037-45.
- Langzieri T M, Parise M S, Siqueira M M et al. Incidence, clinical features and estimated costs of congenital rubella syndrome after a large rubella outbreak in Recife, Brazil, 1999-2000. Pediatr Infect Dis J 2004; 23:1116-22.
- US Centers for Disease Control. Achievements in public health: elimination of rubella and congenital rubella syndrome – United States, 1969-2004. Morb Mort Wkly Rep 2005; 54:1-4.
- Castillo-Solorzano C, Mansigli C, Bravo-Alcantara P et al. Elimination of rubella and congenital rubella syndrome in the Americas. J Infect Dis 2011; 204(Suppl 2):S571-8.
- Law B, MacDonald N, Halperin SA, et al. The Immunization Monitoring Program Active (IMPACT) prospective five year study of Canadian children hospitalized for chickenpox or an associated complication. Pediatr Infect Dis J 2000; 19:1053-9.
- Ziebold C, von Kries R, Lang R, Weigl J, Schmitt HJ. Severe complications of varicella in previously healthy children in Germany: a 1-year study. Pediatrics 2001;108 (5).
- van der Maas NAT, Vermeer-de Bondt PE, de Melker H, Kemmeren JM. Acute cerebellar ataxia in the Netherlands: a study on the association with vaccinations and varicella zoster infection. Vaccine 2009; 27: 1970-3
- Moriuchi H, Rodriguez W. Role of varicella-zoster virus in stroke syndromes. Pediatr Infect Dis J 2000; 19: 648-53.
- Centers for Disease Control. Progress towards interruption of wild poliovirus transmission- worldwide, January 2010 – March 2011. Morb Mort Wkly Rep 2011; 60(18): 582-6.
- Cochi SL, Broome CV, Hightower AW. Immunization of U.S. children with Haemophilus influenzae type b polysaccharide vaccine: a cost-effectiveness model of strategy assessment. JAMA 1985; 253: 521-9.
- Sell SH. Haemophilus influenzae type b meningitis: manifestations and long term sequelae. Pediatr Infect Dis J 1987; 6: 775-8.
- Scheifele DW, Jadavji TP, Law BJ et al. Recent trends in pediatric Haemophilus influenzae type b infections in Canada. Can Med Assoc J 1996; 154: 1041-7.
- Ojo LR, O’Loughlin RE, Cohen AL et al. Global use of Haemophilus influenzae type b conjugate vaccine. Vaccine 2010; 28: 7117-22.
- Scheifele D, Halperin S, Pelletier L et al. Invasive pneumococcal infections in Canadian children, 1991-1998: implications for new vaccination strategies. Clin Infect Dis 2000; 31: 58-64.
- Rajasingham CR, Bonsu BK, Chapman J, Cohen DM, Barson WJ. Serious neurologic sequelae in cases of meningitis arising from infection by conjugate-vaccine related and nonvaccine-related serogroups of Streptococcus pneumoniae. Pediatr Infect Dis J 2008; 27: 771-5.
- Morrow A, De Wals P, Petit G, Guay M, Erickson LJ. The burden of pneumococcal disease in the Canadian population before routine use of the seven-valent pneumococcal conjugate vaccine. Can J Infect Dis Med Microbiol 2007; 18: 121-7.
- Scott JAG. The preventable burden of pneumococcal disease in the developing world. Vaccine 2007; 25: 2398-2405.
- Wong VK, Hitchcock W, Mason WH. Meningococcal infections in children: a review of 100 cases. Pediatr Infect Dis J 1989; 8: 224-7.
- Edwards MS, Baker CJ. Complications and sequelae of meningococcal infections in children. J Pediatr 1981; 99: 540-5.
- Borg J, Christie D, Coen PG, Booy R, Viner RM. Outcomes of meningococcal disease in adolescence: prospective, matched cohort study. Pediatrics 2009;123:e502-509.
- Bettinger JA, Scheifele DW, LeSaux N, et al. The impact of childhood meningococcal serogroup C conjugate vaccine programs in Canada. Pediatr Infect Dis J 2009; 28: 220-4.
- Colditz GA, Brewer TF, Berkey CS, et al. Efficacy of BCG vaccine in the prevention of tuberculosis. JAMA 1994; 271: 698-702.
- Brewer TF. Preventing tuberculosis with Bacillus Calmette-Guerin vaccine: a meta-analysis of the literature. Clin Infect Dis 2000; 31(Suppl 3): S64-7.
- Halperin SA, Wang EEL, Law B, et al. Epidemiological features of pertussis in hospitalized patients in Canada, 1991-1997: report of the Immunization Monitoring Program – Active (IMPACT). Clin Infect Dis 1999; 28: 1238-43.
How to cite this article:
Scheifele DW. Childhood Immunization and Brain Health. In: Tremblay RE, Boivin M, Peters RDeV, eds. Scheifele DW, topic ed. Encyclopedia on Early Childhood Development [online]. https://www.child-encyclopedia.com/immunization/according-experts/childhood-immunization-and-brain-health. Published: April 2013. Accessed November 24, 2025.
Text copied to the clipboard ✓