Childhood Trauma and Adult Stress Responsiveness
Christine Heim, PhD
Emory University, USA
Introduction
The past decades have witnessed an increasing societal awareness of child maltreatment, such as abuse and neglect, which is now considered a public health problem of epidemic dimensions.1 In addition, large numbers of children experience the loss of a parent or live with a mentally ill parent who is likely unable to provide continuous or adequate parental care. Compelling evidence suggests that childhood trauma is a major risk factor for the development of mood and anxiety disorders as well as certain medical diseases, including heart disease and disorders such as chronic fatigue and pain syndromes.2 In adulthood, these disorders often manifest or worsen in relation to acute or chronic life stresses and, importantly, persons with childhood stress experience appear to be sensitized to the adverse effects of subsequent stressors on health.3-4 It appears that adverse experience during development induces vulnerability to the effects of stress later in life and thereby induces risk for developing stress-related disorders.
Subject
The precise mechanism that mediates the effects of early adversity on later stress vulnerability and disease risk has been the subject of intense inquiry in neuroscience research. Studies in rodents and non-human primates have focused on effects of early experience on the structure and function of the brain, including effects at the level of the genome, which may result in altered stress responsiveness. Results suggest that adverse experience, such as maternal separation or low maternal care, induces persistent changes in neural circuits implicated in integration of cognitive and emotional processing, controlling the stress hormone axis and autonomic nervous system, and regulating of arousal and vigilance. These changes produce increased physiologic responses to subsequent stressors, as well as depression-like behaviour, anxiety, cognitive impairment, pain sensitivity, and altered sleep.5-6 It is conceivable that early adverse experience may be causally associated with developing a variety of emotional and bodily disorders, particularly in response to challenge.
Problem
Little is known as to whether findings on the neurobiological effects of early stress observed in animal models can be translated to humans and to what extent such effects may contribute to the development of disorders linked to early stress in epidemiological studies.
Key Research Question
A key question for clinical research concerns is whether adverse experience in childhood is associated with neurobiological changes similar to those observed in animal models and whether these changes are related to disorders such as major depression.
Research Context
Clinical studies conducted in recent years have attempted to identify mechanisms that link childhood trauma to adult disease risk. A primary candidate in investigating this link has been the hypothalamic-pituitary-adrenal (HPA) axis, the organism’s main stress hormone system. At the brain level, a hormone called corticotropin-releasing hormone (CRH) stimulates the HPA axis. The end product of the HPA axis released from the adrenal gland is the stress hormone cortisol. Cortisol exerts multiple effects on metabolism, behaviour, and the immune system that help the organism adapt to challenge. Several brain regions modulate the HPA axis. Brain regions that inhibit the HPA axis are the hippocampus and prefrontal cortex (PFC). The amygdala and noradrenergic fibers from the brain stem activate the stress response. Cortisol in turn shuts off the HPA axis in several parts of the brain. Sustained or increased glucocorticoid (GC) exposure can have adverse effects on the hippocampus, which causes decreases of synapses and decreased production of new neurons. Overexposure to cortisol also negatively affects the PFC. Such damage might progressively reduce the control of the HPA axis and lead to increased stress responses. 7-8
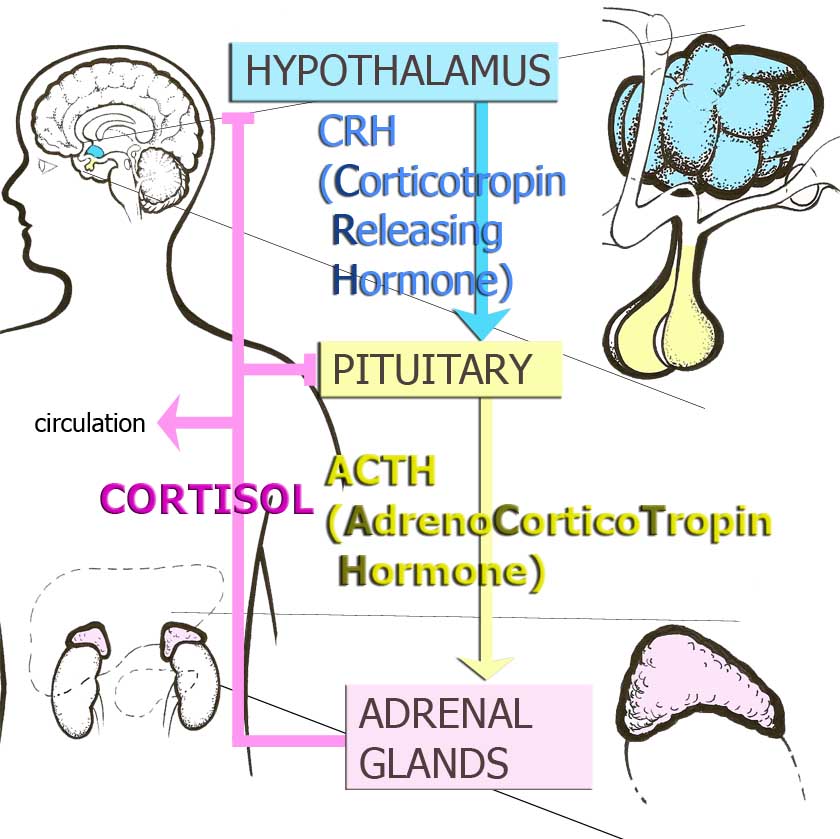
Figure 1. Hypothalamic-Pituitary Adrenocortical Axis or Stress Hormone Axis
CRH neurons also integrate information relevant to stress in several brain areas outside of the hypothalamus. Direct administration of CRH into the brains of animals produces integrated endocrine, autonomic and behavioural responses that parallel signs of stress, depression and anxiety. CRH and another neurotransmitter, norepinephrine, interact in a circuit that connects the amygdala and the hypothalamus with the area in the brain stem called locus coeruleus, in order to regulate vigilance, anxiety and fear, and to integrate endocrine and autonomic responses.9-10
Any disruptions in these systems, as a consequence of early stress, could plausibly lead to altered stress reactivity and the emotional, cognitive and physical changes that are characteristic of disorders related to stress.
Research Results
Retrospective clinical studies in adult humans with childhood trauma histories yielded the following main results:
- Women abused as children exhibit markedly increased stress hormone and heart rate responses to psychosocial laboratory stress, consisting of public speaking and mental arithmetic, compared to controls. The increaseis most pronounced in abused women with current depression.11 Similar results have been reported for adults with early parental loss12, suggesting that results can be applied to other forms of early stress.
- Some abused women, particularly those without depression, exhibit relatively decreased cortisol output under resting conditions13, although findings are not uniformly consistent. Upon further stress, lack of cortisol availability may promote activation of stress systems in the brain, resulting in enhanced stress responsiveness and behavioural changes.
- Lack of regulatory cortisol effects may further be promoted by relative resistance of brain regions to cortisol. Cortisol exerts its effects through special receptors and these receptors can decrease in number or become insensitive. To test this hypothesis, a dexamethasone/CRH test can be applied. Dexamethasone is a synthetic glucocorticoid that suppresses the HPA axis. Subsequent CRH injection induces a rise of cortisol, overriding the suppression, in some persons. This is called an escape. Such escape is the most sensitive marker of HPA axis hyperactivity in depression. Recently, childhood trauma has been associated with marked escape from dexamethasone suppression in adult men, particularly in those with depression, suggesting decreased sensitivity to cortisol’s feedback actions under stimulated conditions.14
- Enhanced autonomic stress reactivity coupled with impaired cortisol sensitivity might enhance immune activation after childhood trauma. Thus, depressed men with high levels of childhood trauma exhibit increased immune activation in response to psychosocial stress, as measured using inflammatory markers.15 Increased inflammatory markers were also associated with childhood adversity in a recent prospective cohort study.16 Messengers in the immune system, such as cytokines, may further stimulate central CRH systems and contribute to risk for several medical diseases, e.g. cardiovascular disease and chronic fatigue.
- The above findings are consistent with increased central CRH activity. Accordingly, levels of CRH in the fluid that surrounds the brain have been found to be associated with perceived childhood stress and abuse experiences. 2,17
- As mentioned before, the hippocampus is one of the most plastic regions of the brain which is critically involved in HPA axis control, explicit memory and context conditioning. Maternal separation and central CRH injections during development alter the structure and plasticity of the hippocampus in laboratory animals. A smaller than normal hippocampus is a hallmark feature of depression. Childhood trauma has been associated with small hippocampi in several studies.18-20 Moreover, small hippocampi in patients with depression have been linked to childhood trauma.21 Repeated bursts of CRH during development and/or increased cortisol reactivity over time may contribute to smaller hippocampi after childhood trauma, leading to further sensitization of stress responses.
- Not all individuals exposed to childhood trauma go on to develop a disorder, even upon further challenge. One approach to understand risk versus resilience is to consider interactions between early stress and dispositional factors, such as genetic variations in neurobiological stress response systems. For example, moderating effects have been demonstrated for variations of genes in various brain systems, including the serotonin and CRH system.22-25 These gene-environment interactions likely reflect genetic moderation of the brain’s functional response to stress.
Research Gaps
Future research should elucidate the neural and molecular basis of increased risk after childhood trauma, and integrate these mechanisms with hormonal findings and clinical symptoms. Studies using functional imaging are needed to develop neural system models of failed adaptation to stress as a consequence of childhood adversity. Interactions between genetic dispositions, gender, and environmental factors in inducing brain changes should be studied. Particular emphasis should be given to studying differential impact of different types of traumas at different developmental stages, in order to identify sources of outcome variability. Such research may identify biological markers of risk and generate precise targets, and time windows of opportunity, for the prevention of adverse outcomes. Longitudinal studies are needed to meet this goal and describe developmental trajectories of adverse outcomes versus resilience.
Conclusions and Implications
In conclusion, results from clinical studies suggest that early stress in humans is associated with long-term neurobiological changes that are comparable to those described in animal studies and suggest sensitization to stress. Genetic variations in stress response systems moderate the link between childhood trauma and adverse outcomes. It must be noted that, in the above studies, changes in stress response systems were only seen in cases with childhood trauma and depression, but not in depressed patients without significant early stress. The implication of these results, taken together, is that several of the classic features of depression may derive from early stress, reflecting vulnerability to develop depression and likely other disorders in response to challenge. This also implies that there may be biologically discernable subtypes of depression and other disorders, as a function of childhood trauma. This notion is also supported by findings of differential treatment responsiveness to psychotherapy versus pharmacotherapy in chronically depressed patients depending on childhood trauma26 and in patients with irritable bowel syndrome.27 Thus, consideration of developmental factors may be useful in improving diagnostic classification of mental and functional somatic disorders and may ultimately help in guiding differential treatment decisions.
References:
- Margolin G, Gordis EB. The effects of family and community violence on children. Annual Review of Psychology 2000; 51:445-479.
- Anda RF, Felitti VJ, Bremner JD, Walker JD, Whitfield C, Perry BD, Dube SR, Giles WH. The enduring effects of abuse and related adverse experiences in childhood. A convergence of evidence from neurobiology and epidemiology. European Archives of Psychiatry and Clinical Neuroscience 2006:256(3):174-186.
- Dougherty LR, Klein DN, Davila J. A growth curve analysis of the course of dysthymic disorder: the effects of chronic stress and moderation by adverse parent-child relationships and family history. Journal of Consulting and Clinical Psychology 2004; 72(6):1012-1021.
- Kendler KS, Kuhn JW, Prescott CA. Childhood sexual abuse, stressful life events and risk for major depression in women. Psychological Medicine 2004; 34:1475-1482.
- Ladd CO, Huot RL, Thrivikraman KV, Nemeroff CB, Meaney MJ, Plotsky PM. Long-term behavioral and neuroendocrine adaptations to adverse early experience. Progress in Brain Research 122: 81-103.
- Meaney MJ, Szyf M. Environmental programming of stress responses through DNA methylation: life at the interface between a dynamic environment and a fixed genome. Dialogues in Clinical Neuroscience 2005;7(2):103-123.
- Arborelius L, Owens MJ, Plotsky PM, Nemeroff CB. The role of corticotropin-releasing factor in depression and anxiety disorders. Journal of Endocrinology 1999;160 (1):1-12.
- Fuchs E, Gould E. Mini-review: in vivo neurogenesis in the adult brain: regulation and functional implications. European Journal of Neuroscience 2000;12(7):2211-2214.
- Owens MJ, Nemeroff CB. Physiology and pharmacology of corticotropin-releasing factor. Pharmacological Review 1991;43(4):425-473.
- Koob GF. Corticotropin-releasing factor, norepinephrine, and stress. Biological Psychiatry 1999;46(9):1167-1180.
- Heim C, Newport DJ, Heit S, Graham YP, Wilcox M, Bonsall R, Miller AH, Nemeroff CB. Pituitary-adrenal and autonomic responses to stress in women after sexual and physical abuse in childhood. JAMA – Journal of the American Medical Association 2000;284(5): 592-597.
- Luecken LJ. Childhood attachment and loss experiences affect adult cardiovascular and cortisol function. Psychosomatic Medicine 1998;60(6):765-772
- Heim C, Newport DJ, Bonsall R, Miller AH, Nemeroff CB. Altered pituitary-adrenal axis responses to provocative challenge tests in adult survivors of childhood abuse. American Journal of Psychiatry 2001;158(4):575-581.
- Heim C, Mletzko T, Purselle D, Musselman DL, Nemeroff CB. The dexamethasone/corticotropin-releasing factor test in men with major depression: role of childhood trauma. Biological Psychiatry 2008;63(4):398-405.
- Pace TWW, Mletzko T, Alagbe O, Musselman DL, Nemeroff CB, Miller AH, Heim C. Increased stress-induced inflammatory responses in male patients with major depression and increased early life stress. American Journal of Psychiatry 2006;163(9):1630-1633.
- Danese A., Pariante CM, Caspi A, Taylor A, Poulton R. Childhood maltreatment predicts adult inflammation in a life-course study. Proceedings of the National Academy of Sciences of the United States of America 2007;104(4):1319-1324.
- Carpenter LL, Tyrka AR, McDougle CJ, Malison RT, Owens MJ, Nemeroff CB, Price LH. Cerebrospinal fluid corticotropin-releasing factor and perceived early-life stress in depressed patients and healthy control subjects. Neuropsychopharmacology 2004 29(4): 777-784.
- Bremner JD, Randall P, Vermetten E, Staib L, Bronen RA, Mazure C, Capelli S, McCarthy G, Innis RB, Charney DS. Magnetic resonance imaging-based measurement of hippocampal volume in posttraumatic stress disorder related to childhood physical and sexual abuse--a preliminary report. Biological Psychiatry 1997;41(1): 23-32.
- Stein MB, Koverola C, Hanna C, Torchia MG, McClarty B, Hippocampal volume in women victimized by childhood sexual abuse. Psychological Medicine 1997; 27(4):951-959.
- Driessen M, Herrmann J, Stahl K, Zwaan M, Meier S, Hill A, Osterheider M, Petersen D. Magnetic resonance imaging volumes of the hippocampus and the amygdala in women with borderline personality disorder and early traumatization. Archives of General Psychiatry 2000; 57(12):1115-1122.
- Vythilingam M, Heim C, Newport DJ, Miller,AH, Vermetten E, Anderson E, Bronen R, Staib L, Charney DS, Nemeroff CB, Bremner JD. Childhood trauma associated with smaller hippocampal volume in women with major depression. American Journal of Psychiatry 2002;159(12):2072-2080.
- Caspi A, Sugden K, Moffitt TE, Taylor A, Craig IW, Harrington H, McClay J, Mill J, Martin J, Braithwaite A, Poulton R. Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science 2003;301(5631):386-389.
- Kendler KS, Kuhn JW, Vittum J, Prescott CA, Riley B. The interaction of stressful life events and a serotonin transporter polymorphism in the prediction of episodes of major depression: a replication. Archives of General Psychiatry 2005. 62(5):529-535.
- Kaufman J, Yang BZ, Douglas-Palumberi H, Houshyar S, Lipschitz D, Krystal JH, Gelernter J. 2004. Social supports and serotonin transporter gene moderate depression in maltreated children. Proceedings of the National Academy of Sciences USA 2004;101(49):17316-1721.
- Bradley RG, Binder EB, Epstein M, Tang Y, Nair H, Liu W, Gillespie CF, Berg T, Evces M, Heim CM, Nemeroff CB, Schwartz A, Cubells JF, Ressler KJ. Influence of child abuse on adult depression: moderation by the corticotropin-releasing hormone receptor gene. Archives of General Psychiatry 2008; 65(2):190-200.
- Nemeroff CB, Heim C, Thase ME, Rush AJ, Schatzberg AF, Ninan PT, Klein DN, McCullough JP, Weiss P, Dunner DL, Rothbaum BO, Kornstein S, Keitner G, Keller MB. Differential responses to psychotherapy versus pharmacotherapy in the treatment of patients with chronic forms of major depression and childhood trauma. Proceedings of the National Academy of Sciences USA 2003. 100(24):14293-14296.
- Creed F, Guthrie E, Ratcliffe J, Fernandes L, Rigby C, Tomenson B, Read N, Thompson DG. Reported sexual abuse predicts impaired functioning but a good response to psychological treatments in patients with severe irritable bowel syndrome. Psychosomatic Medicine 2005; 67:490-499.
How to cite this article:
Heim C. Childhood Trauma and Adult Stress Responsiveness. In: Tremblay RE, Boivin M, Peters RDeV, eds. Encyclopedia on Early Childhood Development [online]. https://www.child-encyclopedia.com/brain/according-experts/childhood-trauma-and-adult-stress-responsiveness. Published: June 2009. Accessed January 27, 2026.
Text copied to the clipboard ✓