[Archived] Routine Immunization in Young Children
Noni E. MacDonald, MD, MSc, FRCPc
Dalhousie University, Canada
Introduction
Our immunization programs for young children are one of the great public health success stories of the twentieth century. They have changed the face of childhood — literally saving the lives of thousands of children every year by minimizing or eliminating the risks of many serious infant and childhood illnesses.1,2 With the exception of safe water, no other modality, not even antibiotics, has had such a major impact on mortality reduction and so improved survival.3
Subject
Recommended Immunization Schedule
The National Advisory Committee on Immunization (NACI) provides the federal government (ie, Health Canada) with ongoing and timely medical, scientific and public health advice relating to immunization.1 The current NACI-recommended immunization schedule for children is summarized in Table 1.1,4 Since health is a provincial not federal responsibility in Canada, each province and territory individually decides which, when and whether specific vaccines will be included in the vaccine program funded by that province or territory.5 Unfortunately, this approach has led to an uneven patchwork of vaccine coverage for children across our land.5,6 For example, as of Dec 2003, the NACI-recommended newer vaccines (eg, varicella,7 conjugated pneumococcal,8 conjugated meningococcal,9 and adolescent acellular pertussis10 vaccines [See below.]) that are only routinely available in some provinces and territories.6 Even though there have been many calls for a National Immunization Strategy in Canada, as yet we do not have one, in contrast to the United States, Australia, the United Kingdom and others.5,6,11-14
Benefits of Routine Immunization among Young Children: Lives Saved
Not so many years ago parents and health care workers alike saw first hand the potential consequences for infants and young children who became infected with the diseases now prevented by routine immunization programs. In the early 1900s, 5 out of every 1,000 children born in Canada and the United States died from pertussis (whooping cough) before they reached their 5th birthday15 and diphtheria (bacteria causing disease that hinders swallowing and breathing) was one of the most common causes of death in children from 1 to 5 years of age, killing thousands of children each year.1 Polio (an infectious viral disease affecting the central nervous system) was a much feared summer scourge that often killed or crippled.16 Table 2 presents a comparison of the prevalence of diseases before and after the introduction of routine vaccines.1,2, 8-10,15-18
To reap the benefit from these vaccines, children must be immunized and immunized on time. These diseases can still kill or maim, even when there is access to modern day intensive care and antibiotic therapy.19-21 In the mid 1990s, many families living in the Russian Federation were retaught the lesson of the dangers of diphtheria and the importance of immunization as diphtheria made a marked resurgence with more than 115,000 cases and 3,000 deaths reported.20 This outbreak occurred in a country where diphtheria had previously been well controlled. The break up of the former USSR led to profound social changes that included a dramatic fall off in immunization rates for infants and children and a failure to give booster doses to adults. Case control studies showed that those who were immunized were protected; those who were not were in trouble.22 This tragic epidemic was due not to vaccine failure, but to a failure to immunize.
Problems
Adverse Events Less Common with Vaccines than with Disease
Table 3 presents the effects of the disease and the known side effects of the vaccine for the routine vaccine-preventable diseases for infants and young children.1,8-10,15-17 In general, all of these diseases are serious and may be fatal, while the vaccine adverse events, if they occur, are usually minor such as local discomfort and/or inflammation at the site of the injection and/or mild fever or rash. Research has shown that the local pain of intramuscular infant immunization with DTaP/IPV/Hib can be diminished by the use of topical lidocaine-prilocaine without adversely affecting the development of the protective response from the multicomponent vaccine23 and the pain of multiple infant injections given during the same visit can be reduced by oral sucrose, oral tactile stimulation (a bottle or pacifier) and parental holding.24
Serious vaccine adverse events occur with the routine immunizations but are a great deal rarer than serious events with the diseases.1,2,8-10,15-17 For example, aseptic meningitis (an infection of the membranes and fluid encasing the brain and spinal cord) occurs in 5% of those who get mumps (a viral disease which causes swelling of the salivary glands in the chin and face) and permanent deafness may occur in up to 0.5%.1,2 In contrast, aseptic meningitis following mumps vaccine with the Jeryl Lynn strain (the type of modified and
weakened mumps viral vaccine strain used in Canada and several other countries) occurs after less than 1/800,000 doses and maybe as low as 1/3,000,000.2,25 Furthermore, the vaccine-associated aseptic meningitis is not followed by permanent problems, like deafness.25
Beyond these known but rare vaccine-associated serious adverse events (Table 3), there have been periodic allegations that infant vaccines may cause other serious problems such as SIDS (sudden infant death syndrome),26 and autism.27 However, research has shown that these claims are unsubstantiated.28-31 There is no causal relationship between infant immunization on one hand and SIDS or autism on the other hand. While an event may have been recognized as happening soon after the receipt of an infant vaccine (ie, establishing a possible temporal relationship), receipt of the vaccine is not the basis for the occurrence of an event.32,33
In order to enhance the evaluation of reported serious vaccine events in Canada, the Advisory Committee on Causality Assessment (ACCA) was set up in 1994 by Health Canada.33 This expert committee is charged with the task of monitoring signals for vaccine safety. The committee regularly reviews all reports of serious and unusual vaccine associated adverse events to determine, through a systematic, standardized approach whether the association of the event to the receipt of the vaccine is likely causal, probably causal, possibly causal, unlikely causal, unrelated or unclassifiable.33 On the international level, the World Health Organization set up the Global Advisory Committee on Vaccine Safety in 1999 whose task is to respond promptly, efficiently and with scientific rigor to vaccine safety issues of potential global importance.34
In 1991, to improve the detection of serious vaccine-associated adverse events, vaccine failures and selected infant and child infectious diseases that are now or are soon to be vaccine preventable, Health Canada, in collaboration with the Canadian Paediatric Society and others, piloted a cross-Canada paediatric hospital-based active surveillance network in 5 centres. The network was then expanded to 11 centres in 1995 and 12 centres in 1999 (IMPACT).35-37 Compiled network data has repeatedly shown that the routine vaccines for young children are very safe.38 In addition, IMPACT has proven valuable in detecting rare but unexpected serious events (eg, disseminated Bacille Calmette-Guerin (BCG) infections in aboriginal infants immunized with BCG39) that have lead to policy reevaluation.40 IMPACT has also been able to show a sharp decline in disease following the introduction of a new vaccine41 and a decrease in side effects following a shift to a new, improved vaccine.42
Research Context
Best Practices for Vaccine Programs
In 1995, NACI initiated a 2-year consultative process to develop guidelines for childhood immunization practicesapplicable to both the public and the private systems of vaccine delivery in Canada. Table 4 provides a brief overview of the guidelines.1 Research has shown that a number of factors can enhance vaccine uptake, including timely reminders, quality parent education materials, after-hours and weekend clinics, vaccine uptake monitoring, multiple vaccines given during one visit, standing orders for vaccines, multi-component provider education, and the elimination of financial barriers to immunization.1,5,6,11, 43-47
When it comes to giving consent for immunization, research has shown that what matters to parents is that they receive the information they need to make an informed decision,46,48-50 but the mode in which this information is given does not matter.49 Bearing this in mind, the 2002 edition of the NACI Canadian Immunization Guide was expanded to include a separate chapter on consent issues and parental concerns regarding immunization to help health care providers better counsel parents.1 Recognizing that the information on vaccines contained in the NACI Canadian Immunization Guide may be too technical for many parents, the Canadian Paediatric Society supported the development of a vaccine handbook specifically designed for parents entitled, “Your Child’s Best Shot,” which was first published in 1997, then updated to include the newer NACI-recommended vaccines in 2002.2
As noted above, well-informed health care providers are an important factor in enhanced vaccine uptake, but research has shown that many are not well informed.45,46,50,51 Efforts to improve on this knowledge deficit include the revamping and upgrading of the NACI Canadian Immunization Guide, continuing vaccine education events for doctors and nurses, journal articles, further research, the formation of the Canadian Coalition for Immunization Awareness and Promotion,52 the formation of the Canadian Association for Immunization Research and Evaluation53 and the biannual National Immunization Conference.54
Vaccine Programs for Young Children with Special Needs
While the routine NACI vaccine schedule (Table 1) is appropriate for the majority of Canadian children there are subgroups with special needs including:
- infants and young children born outside of Canada who come as immigrants, refugees or foreign adoptees who may not have received all of the vaccines recommended in Canada, and/or may not have adequate vaccine documentation.
- infants born prematurely
- infants and children who are immunocompromised from birth or from disease
- infants and children who have bleeding disorders or have a nonfunctional or absent spleen
- infants and young children who travel to other countries.1 In each of these cases, the routine immunization requirements and schedule may need to be adapted.1, 55, 56
Recent Research Results
Newer NACI-recommended Vaccines for Young Children
There are 3 vaccines recently recommended by NACI for infants and young children which are not yet covered by funded vaccine programs in all of the provinces and territories.6 These include the varicella vaccine for prevention of chicken pox,7 conjugated pneumococcal vaccine for the prevention of blood infection, pneumonia and meningitis due to pneumococcal bacteria,8 and conjugated meningococcal vaccine for the prevention of meningitis and blood infection, again, due to this organism.9 The risks of these diseases, vaccine benefits and side effects are summarized in Tables 2 and 3. In all three cases these vaccines have been shown to be safe and effective in preventing serious diseases in infants and young children, but each is also relatively expensive compared to the cost of the “regular” infant immunizations.57 The prohibitive costs of these vaccines has led to delays and disparities in having these vaccines added to the “routine” list covered by each province and territory.5,6,47 A similar problem exits for the acellular pertussis vaccine for adolescents and adults. While acellular pertussis vaccine is available across Canada for infants and young children, despite NACI recommendations, the acellular vaccine for adolescents and adults is not yet routinely available across the country. Widespread use of this vaccine in adolescents and adults has the potential to decrease pertussis in families and thus decrease exposure of infants who are too young to be immunized (ie, less than 6 weeks of age), the group at highest risk for fatal disease.58
Conclusions
The NACI-recommended vaccines for young children are a safe and effective means of eliminating or minimizing the risks of many serious infant and childhood illnesses. Infants and children who are not immunized continue to be at risk. The NACI Canadian Guide to Immunization1 is the best detailed source of information on all aspects of immunization for health care providers and Your Child’s Best Shot2 provides quality information for parents.
Implications
A National Immunization Program is needed to improve equity of access across this country to all of the NACI-recommended vaccines for infants and young children in order to be able to protect all of our children from the potential damage incurred by a vaccine-preventable disease. Not ensuring equity of access means many infants and children remain at risk for problems such as acquired deafness from meningitis due to pneumococal infection, along with its profound developmental implications. Policy makers at the federal, provincial, and territorial levels must work together to ensure a National Immunization Program for Canadian infants and children so that all have access to NACI-recommended vaccines.
TABLE 1
NACI-recommended Immunization Schedule for Infants and Children (from references 1, 8-10, 15-18)
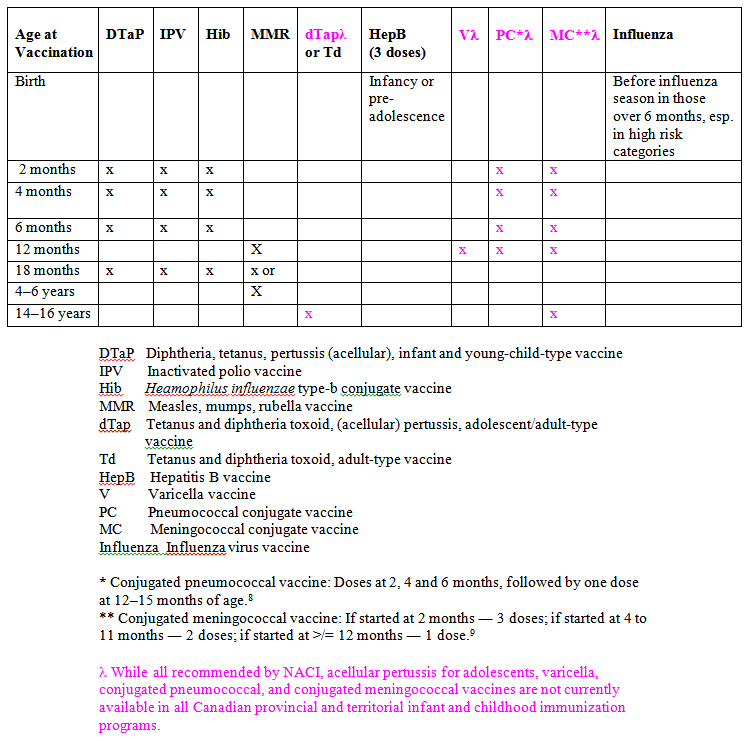
TABLE 2
Serious Illnesses in Infants, Children and Youth in Canada in the Pre- and Post-Vaccine Eras (from references 1, 2, 8-10,15-18)
| Disease/ Organism | Incidence Before Vaccination | Incidence After Vaccination |
|---|---|---|
| Polio 3 types of polio virus |
2.5 to 28.3/100,000 Epidemics: up to 20,000 cases of paralysis. |
Disease eradicated from Canada and from most countries in the world. |
| Diphteria |
In 1924, 9,000 cases reported. Major cause of death in 1 to 5 year olds. |
No cases reported since 1996, and prior to this only 2–5 per year. |
| Tetanus (lock jaw) |
60 to 75 cases per year, with 40 to 50 deaths |
Less than 2 cases per year in past 15 years. |
| Pertussis (whooping cough) |
Over 150/100,000 cases per year with 50 to 100 deaths. |
10/100,000 cases per year with 1 to 3 deaths in very young infants. |
| Haemophilus influenzae type b |
Overall 2,000 cases per year with 1500 in those <5 years of age. Leading cause of bacterial meningitis in infancy. |
Less than 50 cases per year. Rare cause of bacterial meningitis in infants. |
| Measles |
Cyclic epidemics every 2-3 years. 300,000–400,000 cases per year. |
Now fewer than 400 cases per year. |
| Mumps |
About 30,000 reported cases per year but many more not reported |
Less than 500 cases per year. |
| Rubella |
About 250,000 cases per year, with over 200 congenital rubella syndrome (CRS)/year |
Less than 100 cases reported per year, 1 to 2 congenital rubella syndrome (CRS) /year. |
| Influenza |
Yearly epidemics with up to 20% of the population being infected. Wide variation in annual incidence. Last major outbreak: 1968 with 50 million cases, 33,000 deaths. |
Since influenza virus changes on a yearly basis, yearly vaccination is required. Current program is aimed at high risk with limited uptake by others. With well-matched vaccine–influenza in community, 70–90% of illness is prevented, if immunocompetent. If ill-matched, it is only 30–60% protective. Less effective if not immunocompetent. |
| Hepatitis B |
20,000 new infections per year, 1 in 200 people in population is a chronic carrier. BC had a rate of 33.7/100,000 in 1992.Risk of transmission from an infected mother to her newborn infant is 90%. |
From 1992 to 2002 in BC after adopting a Grade 6 vaccine program, overall rate acute infection fell from 7 to 2/100,000 and in 12 to 21 year olds from 1.7 to 0/100,000. Immunization of newborn infants prevents transmission from mother in > 90% of cases. |
| Varicella (Chicken pox) |
Infection in 50% of children by age 5 and 90% by age 12. |
Varicella mortality in the US has decreased by 76% with national program and coverage rates of 80% in less than 3 year olds. |
| Streptococcus pneumoniae |
About 500,000 cases of pneumococcal diseases per year with over 200,000 in children under 5 years of age. Rate of invasive disease <5 years 35–64/100,000; < 2 years 59–112/100,000. |
Conjugated heptavalent vaccine for infants only licensed in Canada in 2001.In the US, clinical trials in infants showed vaccine efficacy of 94% for invasive diseases due to strains in vaccine and 89% for invasive disease due to any pneumococcal strain. |
| Neisseria meningitidis |
Endemic in Canada with epidemics every 10 to 15 years. 200 to 350 endemic cases per year. Rates in 3 highest age groups: |
Conjugated group C vaccine for infants only licensed in Canada in 2001.In UK, routine infant immunization started in 1999 with follow up campaign for children and adolescents has decreased disease by >90%. |
TABLE 3
Comparison of Effects of Serious Infant and Childhood Diseases and Adverse Effects of Vaccines (from references 1, 2, 8-10, 15-17)
| Disease / Organism |
Effects of Disease |
Side Effects of Vaccines * |
|
Polio |
4–8% have minor illness, 1% get severe disease- paralytic polio, 1 in 20 hospitalized patients die and 50% remain paralyzed. |
Local discomfort or redness at the site of injection in 5%. Killed vaccine so no risk of vaccine-associated polio. |
|
Diphteria |
5–10% of cases die even with ICU care, antitoxin and antibiotics. The toxin may lead to neurological and cardiac complications. |
DTaP vaccine: Local discomfort, swelling and /or redness at the site of injection in 20%, fever in 5%. A transient nodule may occur at the injection site, lasting for a few weeks. Up to 70% develop redness and swelling at the 4-6yr booster. |
|
Tetanus (lock jaw) |
10% of cases die, even with ICU care, antitoxin and antibiotics. Risk is greatest for the very young and the very old. |
See above for DTaP. Local reactions increase with age, esp. in adults with Td boosters. Peripheral nerve damage has rarely been reported (<1/1,000,000). |
|
Pertussis (whooping cough) |
1/400 infants with pertussis die, 1/400 sustain permanent brain damage. If under 6 months, 1% of cases die from pneumonia or fatal oxygen deprivation of the brain. |
As above for DTaP. Far fewer side effects with the acellular pertussis (aP) vaccine than the previous whole-cell pertussis vaccine (P). |
|
Haemophilus influenzae type b |
5% of cases of meningitis die, 10–15% have permanent brain damage and 10–20% have deafness. |
Usually in combination, as with DTaP/IPV/Hib. See above for side effects (same as for DTaP). |
|
Measles |
10% have complications such as pneumonia, ear infections. 1/1,000 have encephalitis (infection of the brain) with 10% dying and 25% being left with permanent brain damage, 1/25,000 have SSPE (a delayed fatal degenerative brain disease. |
Usually in combination, as with MMR. 5–10% have discomfort or local swelling and fever, with or without a rash.1/24,000 have low platelets<1/1,000,000 have encephalitis. |
|
Mumps |
1/20 develop aseptic meningitis (viral infection of tissues and fluids around the brain). 1/200 develop encephalitis. 1/200,000 are left deaf. Inflammation of testicles in 20–30% of males; inflammation of ovaries in 5% of post-pubertal females. |
Local discomfort, swelling and redness or fever in 5–10%. 1% develop parotitis (swelling of the largest salivary gland, the parotid). 1 in 3 million develop aseptic meningitis. |
|
Rubella |
50% have rash, swollen glands, fever; 50% of adolescents and adults have arthritis and arthralgias; 1/6,000 have encephalitis. In the first 10 weeks of pregnancy, 85% risk of congenital rubella syndrome causes death of fetus, deafness, blindness and/or heart disease. |
10% have local discomfort and fever, 5% have swollen glands, arthralgias (esp. adults), stiff neck. 1% develop noninfectious rash. |
|
Influenza |
Highest mortality rate in those over 65 years and in infants aged <12 months. Complications: pneumonia, febrile seizures, encephalitis, myocarditis, and myositis, Reye’s syndrome. |
Local mild reactions at injection site and/or low fever for 1 to 2 days in up to 60%. Occasional mild oculorespiratory syndrome. Rare: Guillian-Barre syndrome 1/1,000,000. |
|
Hepatitis B |
Variable: asymptomatic to overwhelming liver disease. Neonate asymptomatic, 5–15% of 1 to 5 year olds have symptoms, 33–50% older children egg nausea, jaundice, fever, vomiting, big liver, spleen. |
15% experience local discomfort and occasionally experience low-grade fever. |
|
Varicella (chicken pox) |
Death rate 1–3 /100,000 cases in children. Complications in 5–10% of previously healthy children: pneumonia, encephalitis (1/5,000), cerebellar ataxia (1/4,000), osteomyelitis, hepatitis, septic arthritis. In 50% of children who get flesh-eating disease (necrotizing fascitis), chicken pox precedes it. Shingles in adults. Congenital varicella syndrome. |
15–20% experience mild swelling, discomfort at injection site and/or fever. 1–5% develop mild rash. |
|
Streptococcus pneumoniae |
Leading cause of invasive bacterial disease in young children. Annual cases: 65 meningitis (hearing loss 20–30%, brain damage 15–20%), 700 cases bacteremia, 2,200 cases hospitalized with pneumonia, 9,000 cases non-hospitalized pneumonia. Case fatality rate <6 months 4.3%, 12 years 2%. 15 deaths/year in <5 years. Sickle cell disease, HIV more at risk bad disease. |
Heptavalent infant/ toddler conjugate vaccine well tolerated. Mild local reactions from 10–15%. |
|
Neisseria meningitidis |
Meningitis 30–50% (MR 5%), meningitis + bacteremia 40%, bacteremia alone 7–10% (MR 20-40%). Other complications: arthritis, pneumonia, peritonitis. Case fatality rate 10% despite ICU/antibiotics. Highest mortality rate (MR): <1 year 1/100,000 |
Infant/toddler conjugate C vaccine: local mild reactions less common than with DTaP/IPV/Hib, severe reactions are very rare. |
* Anaphylaxis, a potentially life-threatening allergic reaction, occurs rarely (0.11 to 0.31 reports per 100,000 doses of vaccine distributed). It is rarer in infants and young children and occurs within 30 minutes of receipt of vaccine. Can be treated with an injection of epinephrine.
TABLE 4
National Guidelines for Childhood Immunization Practices: Summary*
| 1 |
Immunization services should be readily available. |
| 2 |
There should be no barriers or unnecessary prerequisites to the receipt of vaccines. |
| 3 |
Providers should use all clinical encounters to screen for needed vaccines and, when indicated, vaccinate children. |
| 4 |
Providers should educate parents in general terms about immunization. |
| 5 |
Providers should inform parents in specific terms about the risks and benefits of the vaccines their child is to receive. |
| 6 |
Providers should recommend deferral or withholding of vaccines for true contraindications only. |
| 7 |
Providers should administer all vaccine doses for which a child is eligible at the time of each visit. |
| 8 |
Providers should ensure that all vaccinations are accurately and completely recorded. |
| 9 |
Providers should maintain easily retrievable summaries of the vaccination records to facilitate age-appropriate vaccination. |
| 10 |
Providers should report clinically significant adverse events following vaccination promptly, accurately, and completely. |
| 11 |
Providers should report all cases of vaccine-preventable diseases as required under provincial and territorial legislation. |
| 12 |
Providers should adhere to appropriate procedures for vaccine management. |
| 13 |
Providers should maintain up-to-date, easily retrievable protocols at all locations where vaccines are administered. |
| 14 |
Providers should be properly trained and maintain ongoing education regarding current immunization recommendations. |
| 15 |
Providers should operate a tracking system. |
| 16 |
Audits should be conducted in all immunization clinics to assess the quality of immunization records and assess immunization coverage levels. |
*Adapted from the Canadian Immunization Guide, 6th edition.1
References
- National Advisory Committee on Immunization. Canadian Immunization Guide. 6th ed. Ottawa, Ontario: Health Canada; 2002. Available at: http://dsp-psd.communication.gc.ca/Collection/H49-8-2002E.pdf .. Accessed July 22, 2004.
- Gold R. Your child’s best shot: a parent’s guide to vaccination. 2nd ed. Ottawa, Ontario: Canadian Paediatric Society; 2002.
- Plotkin SL, Plotkin SA. A short history of vaccination. In: Plotkin SA, Orenstein WA, eds. Vaccines. 3rd ed. Philadelphia, Pa: WB Saunders Co; 1999:1-12.
- Division of Immunization and Respiratory Diseases. Population and Public Health Branch. Health Canada. Immunization Schedule. Available at: http://www.phac-aspc.gc.ca/im/index.html. Accessed July 22, 2004.
- Naus M, Scheifele DW. Canada needs a national immunization program: an open letter to the Honourable Anne McLellan, federal minister of health. CMAJ - Canadian Medical Association Journal 2003;168(5):567-568.
- Infectious Diseases and Immunization Committee, Canadian Paediatric Society. Routine immunization schedule: Update 2004. Peadiatric Child Health 2004;9(1):17-20. Available at: http://www.cps.ca/english/statements/ID/PIDNoteImmunization.htm. Accessed July 22, 2004.
- National Advisory Committee on Immunization. Update on varicella. CCDR - Canadian Communicable Disease Report 2004;30(ACS-1):1-28. Available at: http://www.phac-aspc.gc.ca/publicat/ccdr-rmtc/04pdf/acs-dcc-30-1.pdf.. Accessed July 22, 2004.
- National Advisory Committee on Immunization. Statement on recommended use of pneumococcal conjugate vaccine. CCDR – Canada Communicable Disease Report 2002;28(ACS-2):1-32. Available at: http://www.phac-aspc.gc.ca/publicat/ccdr-rmtc/02pdf/acs28-2.pdf. Accessed July 22, 2004.
- National Advisory Committee on Immunization. Statement on recommended use of meningococcal vaccines. CCDR – Canada Communicable Disease Report 2001;27(ACS-6):2-36. Available at: http://www.phac-aspc.gc.ca/publicat/ccdr-rmtc/01pdf/acs27-5-6.pdf Accessed July 22, 2004.
- National Advisory Committee on Immunization. Prevention of pertussis in adolescents and adults. CCDR – Canada Communicable Disease Report 2003;29(ACS-5):1-9. Available at: http://www.phac-aspc.gc.ca/publicat/ccdr-rmtc/03pdf/acs-dcc-29-5-6.pdf . Accessed July 22, 2004.
- Lynk A. A call to arms (and legs): Implement the National Immunization Strategy. Peadiatrics and Child Health 2002;7(2):615-616.
- Romanow RJ, commisionner. Building on values: The future of health care in Canada. Final report. Saskatoon, Saskatchewan: Commission on the Health Care in Canada; 2002. Available at: http://www.hc-sc.gc.ca/english/care/romanow/hcc0086.html. Accessed July 22, 2004.
- National Immunization Strategy. In: Budget 2003. Investing in Canada’s Health Care System. Canada: Department of Finance; 2003:15. Available at: http://www.fin.gc.ca/budget03/booklets/bkheae.htm. Accessed July 22, 2004.
- National Advisory Committee on SARS and Public Health. Learning from SARS - Renewal of public health in Canada. Ottawa: Health Canada; 2003. Available at: http://www.phac-aspc.gc.ca/sars-sras/index.html. Accessed July 22, 2004.
- Cherry JD, Brunell PA, Golden GS, Karson DT. Report of the task force on pertussis and pertussis immunization. Pediatrics 1988;81(suppl):939-984.
- Rutty CJ. The middle-class plague: epidemic polio and the Canadian state, 1936-1937. Bulletin Canadien d'Histoire de la Medecine / Canadian Bulletin of Medical History 1996;13(2):277-314. Available at: http://www.healthheritageresearch.com/MCPlague.html. Accessed July 23, 2004.
- Patrick DM, Bigham M, Ng H, White R, Tweed A, Skowronski DM. Elimination of acute hepatitis B among adolescents after one decade of an immunization program targeting Grade 6 students. Pediatric Infectious Disease Journal 2003;22(10):874-877.
- Immunization and Respiratory Infections Division, Centre for Infectious Disease Prevention and Control. Enhanced surveillance of invasive meningococcal disease in Canada: 1 January, 1999, through 31 December, 2001. CCDR - Canada Communicable Disease Report 2004;30(3):17-28. Available at: http://www.phac-aspc.gc.ca/publicat/ccdr-rmtc/04vol30/dr3003ea.html . Accessed July 23, 2004.
- Samba E, Nkrumah F, Leke R. Getting polio eradication back on track in Nigeria. New England Journal of Medicine 2004;350(7):645-646.
- Markina SS, Maksimova NM, Vitek CR, Bogatyreva EY, Monisov AA. Diphtheria in the Russian Federation in the 1990s. Journal of Infectious Diseases 2000;181(Suppl 1):S27-S34.
- Grewal S, Scheifele D. Haemophilus influenzae type b disease at 11 pediatric centres, 1996-1997. CCDR - Canada Communicable Disease Report 1998;24(13):105-108. Available at: http://www.phac-aspc.gc.ca/publicat/ccdr-rmtc/98vol24/dr2413ea.html . Accessed July 23, 2004.
- Bisgard KM, Rhodes P, Hardy IRB, Litkina IL, Filatov NN, Monisov AA, Wharton M. Diphtheria toxoid vaccine effectiveness: A case controlled study in Russia. Journal of Infectious Diseases 2000;181(Suppl 1):S184-S187.
- Halperin BA, Halperin SA, McGrath P, Smith B, Houston T. Use of lidocaine-prilocaine patch to decrease intramuscular injection pain does not adversely affect the antibody response to diphtheria-tetanus-acellular pertussis-inactivated poliovirus-Heamophilus influenzae type b conjugate and hepatitis B vaccines in infants from birth to six months of age. Pediatric Infectious Disease Journal 2002;21(5):399-405.
- Reis EC, Roth EK, Syphan JL, Tarbell SE, Holubkov R. Effective pain reduction for multiple immunization injections in young infants. Archives of Pediatrics and Adolescent Medicine 2003;157(11):1115-1120.
- Plotkin SA, Wharton M. Mumps Vaccine. In: Plotkin SA, Orenstein WA, eds. Vaccines. 3rd ed. Philadelphia, Pa: WB Saunders Co.; 1999:279-281.
- Baraff LJ, Ablon WJ, Weiss RC. Possible temporal association between diphtheria-tetanus toxoid-pertussis vaccination and sudden infant death syndrome. Pediatric Infectious Disease Journal 1983;2(1):7-11.
- Wakefield AJ, Murch SH, Anthony A, Linnell J, Casson DM, Malik M, Berlowitz M, Dhillon AP, Thomson MA, Harvey P, Valentine A, Davies SE, Walker-Smith JA. Ileal-lymphoid-nodular hyperplasia, non-specific colitis, and pervasive developmental disorder in children (Retracted article. See vol 363, pg 750, 2004). Lancet 1998;351(9103):637-641.
- Howson CP, Howe CJ, Fineberg HV, eds; Committee to Review the Adverse Consequences of Pertussis and Rubella Vaccines, Institute of Medicine. Adverse effects of pertussis and rubella vaccines. Washington, DC: National Academies Press; 1991.
- Strauss B, Bigham M. Does measles-mumps-rubella (MMR) vaccination cause inflammatory bowel disease and autism? CCDR - Canada Communicable DiseaseReport 2001;27(8):65-72. Available at: http://www.phac-aspc.gc.ca/publicat/ccdr-rmtc/01vol27/dr2708ea.html . Accessed July 23, 2004.
- Stratton K, Gable A, Shetty P, McCormick M, eds. Immunization Safety Review: Measles-Mumps-Rubella Vaccines and Autism. Washington, DC: Institute of Medicine, National Academies Press; 2001. Available at: http://www.nap.edu/books/0309074479/html/. Accessed July 23, 2004.
- Wilson K, Mills E, Ross C, McGowan J, Jadad A. Association of autistic spectrum disorder and the measles, mumps and rubella vaccine. A systematic review of current epidemiological evidence. Archives of Pediatrics and Adolescent Medicine 2003;157(7):628-634.
- Stratton KR, Howe CJ, Johnston RB, eds. Adverse Events Associated with Childhood Vaccines. Evidence Bearing on Causality. Washington, DC: Institute of Medicine. National Academies Press; 1994.
- Collett JP, MacDonald N, Cashman N, Pless R, Advisory Committee on Causality Assessment. Monitoring signals for vaccine safety: the assessment of individual adverse event reports by an expert advisory committee. Bulletin of the World Health Organization 2000;78(2):178-185.
- World Health Organization. Global Advisory Committee on Vaccine Safety (GACVS). Available at: http://www.who.int/vaccine_safety/en/. Accessed July 26, 2004.
- Canadian Paediatric Society. IMPACT - Immunization Monitoring Program - ACTive. Available at: http://www.cps.ca/English/surveillance/IMPACT/IMPACT.htm Accessed July 26, 2004.
- Morris R, Halperin SA, Dery P, Mills E, Lebel M, MacDonald N, Gold R, Law B, Jadavji T, Scheifele DW, Marchessault V, Duclos P: IMPACT monitoring network: A better mousetrap. Canadian Journal of Infectious Diseases 1993;4(4):194-195.
- Scheifele DW, Halperin SA, CPS/Health Canada, Immunization Monitoring Program, Active (IMPACT). Immunization Monitoring Program, Active: a model of active surveillance of vaccine safety. Seminars in Pediatric Infectious Diseases 2003;14(3):213-219.
- Scheifele DW, Halperin SA, Gold R, Samson H, King A, Canadian Paediatric Society/Health Canada Immunization Monitoring Program, ACtive (IMPACT). Assuring vaccine safety: a celebration of 10 years of progress with the IMPACT project. Paediatrics and Child Health 2002;7(9):645-648. Available at: http://www.pulsus.com/Paeds/07_09/sche_ed.htm. Accessed July 26, 2004.
- Scheifele D, Law B, Jadavji T, on behalf of IMPACT. Disseminated Bacille Calmette-Guerin Infection: Three recent Canadian cases. CCDR - Canada Communicable Disease Report 1998;24(9):69-75. Available at: http://www.phac-aspc.gc.ca/publicat/ccdr-rmtc/98vol24/dr2409ea.html . Accessed July 26, 2004.
- Elwood K. Bacille Calmette-Guérin vaccination. In: Long R, ed. Canadian Tuberculosis Satandards. 5th ed. Ottawa, Ontario: Health Canada, Canadian Lung Association; 2000: 223-228. Available at: http://www.phac-aspc.gc.ca/publicat/cts-ncla00/pdf/cts00.pdf. Accessed July 26, 2004.
- Scheifele D, Halperin S, on behalf of IMPACT. Haemophilus influenzae type b disease control using Pentacelä, Canada, 1998-1999. CCDR - Canada Communicable Disease Report 2000;26(11):93-96. Available at: http://www.phac-aspc.gc.ca/publicat/ccdr-rmtc/00vol26/dr2611ea.html . Accessed July 26, 2004.
- Le Saux N, Barrowman NJ, Moore DL, Whiting S, Scheifele D, Halperin S, for Members of the Canadian Paediatric Society/ Health Canada Immunization Monitoring Program–Active (IMPACT). Decrease in hospital admissions for febrile seizures and reports of hypotonic-hyporesponsive episodes presenting to hospital emergency departments since switching to acellular pertussis vaccine in Canada: a report from IMPACT. Pediatrics 2003;112(5):e348-e348. Available at: http://www.pediatrics.org/cgi/content/full/112/5/e348. Accessed July 26, 2004.
- DuPlessis HM, Bell WC, Boulter SC, Cora-Bramble D, Feild C, Handal GA, Katcher ML, Rushton FE, Wood DL, Lavin A, Melinkovich P, Belardo JH, Rodewald LE, Varrasso DA, Mejia CA, Yasuda KE, Hammer LD, Harbaugh NR, Itkin PG, Jakubec PJ, Walker RD, France FL, Herr TJ, Lieberthal AS, Swanson J, Grimm KT, Bien AA, Davis T, Price WS, Sebring RH. Increasing immunization coverage. Pediatrics 2003;112(4):993-996.
- Bjornson GL, Scheifele DW, Lajeunesse C, Bell A. Effect of reminder notices on the timeliness of early childhood immunizations. Paediatrics and Child Health 1999;4(6):403-408.
- Boulianne N, Deceuninck G, Duval B, Lavoie F, Dionne M, Carsley J, Valiquette L, Rochette L, De Serres G. Why are some children incompletely vaccinated at the age of 2? [in French]. Canadian Journal of Public Health. Revue Canadienne de Sante Publique 2003;94(3):218-223.
- de Courval FP, De Serres G, Duval B. Varicella vaccine: factors influencing uptake. Canadian Journal of Public Health. Revue Canadienne de Sante Publique 2003;94(4):268-271.
- Scheifele DW. New vaccines and the rising costs of caring. Paediatrics and Child Health 2000;5(7):371-372. Available at: http://www.pulsus.com/Paeds/05_07/sche_ed.htm. Accessed July 26, 2004.
- Ball LK, Evans G, Bostrom A. Risky buisness: Challenges in vaccine risk communication. Pediatrics 1998;101(3):453-458.
- Bjornson GL, Scheifele DW, Gold R. Assessment of parent education methods for infant immunization. Canadian Journal of Public Health. Revue Canadienne de Sante Publique 1997;88(6):405-408.
- Gust DA, Woodruff R, Kennedy A, Brown C, Sheedy K, Hibbs B. Parental perceptions surrounding risks and benefits of immunization. Seminars in Pediatric Infectious Diseases 2003;14(3):207-212.
- Dionne M, Boulianne N, Duval B, Lavoie F, Laflamme N, Carsley J, Valiquette L, Gagnon S, Rochette L, De Serres G. Lack of conviction about vaccination in certain Quebec vaccinators [in French]. Canadian Journal of Public Health. Revue Canadienne de Sante Publique 2001;92(2):100-104.
- The Canadian Coalition for Immunization Awareness and Promotion / Coalition canadienne pour la sensibilisation et la promotion de la vaccination. Available at : http://www.immunize.cpha.ca. Accessed July 26, 2004.
- The Canadian Association for Immunization for Research and Evaluation / Association canadienne pour la recherche et l’évaluation en immunisation. Available at: http://www.caire.ca/. Accessed July 26, 2004.
- Health Canada. Proceedings of the Canadian National Immunization Conference. Canada’s National Immunization Strategy: From Vision to Action. Victoria BC. Dec1-3, 2002. CCDR - Canada Communicable Disease Report 2003;29(S4):1-24. Available at: http://www.phac-aspc.gc.ca/publicat/ccdr-rmtc/03pdf/29s4e.pdf . Accessed July 26, 2004.
- Health Canada. Population and Public Health Branch. Travel Medicine Program. Available at: http://www.travelhealth.gc.ca. Accessed July 26, 2004.
- Children and youth new to Canada: A health care guide. Ottawa, Ontario: Canadian Paediatric Society; 1999.
- Tengs TO, Adams ME, Pliskin JS, Safran DG, Siegel JE, Weinstein MC, Graham JD. Five-hundred life-saving interventions and their cost-effectiveness. Risk Analysis 1995;15(3):369-390.
- Mikelova LK, Halperin SA, Scheifele D, Smith B, Ford-Jones E, Vaudry W, Jadavji T, Law B, Moore D, members of the Immunization Monitoring Program, Active (IMPACT). Predictors of death in infants hospitalized with pertussis: a case-control study of 16 pertussis deaths in Canada. Journal of Pediatrics 2003;143(5):576-581.
How to cite this article:
MacDonald NE. [Archived] Routine Immunization in Young Children. In: Tremblay RE, Boivin M, Peters RDeV, eds. Scheifele DW, topic ed. Encyclopedia on Early Childhood Development [online]. https://www.child-encyclopedia.com/immunization/according-experts/routine-immunization-young-children. Published: August 2004. Accessed February 25, 2026.
Text copied to the clipboard ✓