Stress and Early Brain Development
Megan R. Gunnar, PhD, Adriana Herrera, MA, Camelia E. Hostinar, BS
University of Minnesota, USA
Introduction
Stress is a condition in which an individual experiences challenges to physical or emotional well-being that overwhelm their coping capacity. While some experience with manageable stress is important for healthy development, prolonged, uninterrupted, overwhelming stress can have toxic effects. This type of toxic stress is often associated with childhood abuse and neglect.
In the early years of life when the brain is developing rapidly it is particularly sensitive to environmental influences. Toxic early life stress (ELS) may induce persistent hyper-sensitivity to stressors and sensitization of neural circuits and other neurotransmitter systems which process threat information. These neurobiological sequelae of ELS may promote the development of short and long-term behavioural and emotional problems that may persist and increase the risk for psychopathology and physical health disorders into adulthood.1,2
Subject
Research has begun to identify the neural circuits, brain structures, and endocrine systems affected by ELS and their role in emergent psychopathology and medical problems. Multidisciplinary research in the areas of risk and resilience, developmental psychopathology, psychoneuroendocrinology, neuroscience, and molecular and behavioural genetics has elucidated factors that increase vulnerability to stressors and those which protect children from their deleterious effects. Understanding the mechanisms through which ELS “gets under the skin” should help us to identify intervention and prevention targets, thus having broad implications for policy and practice.
Problems
The stress response system involves the sympathetic nervous system, the various neurotransmitter systems, the immune system, and the hypothalamic-pituitary adrenocortical (HPA) axis.
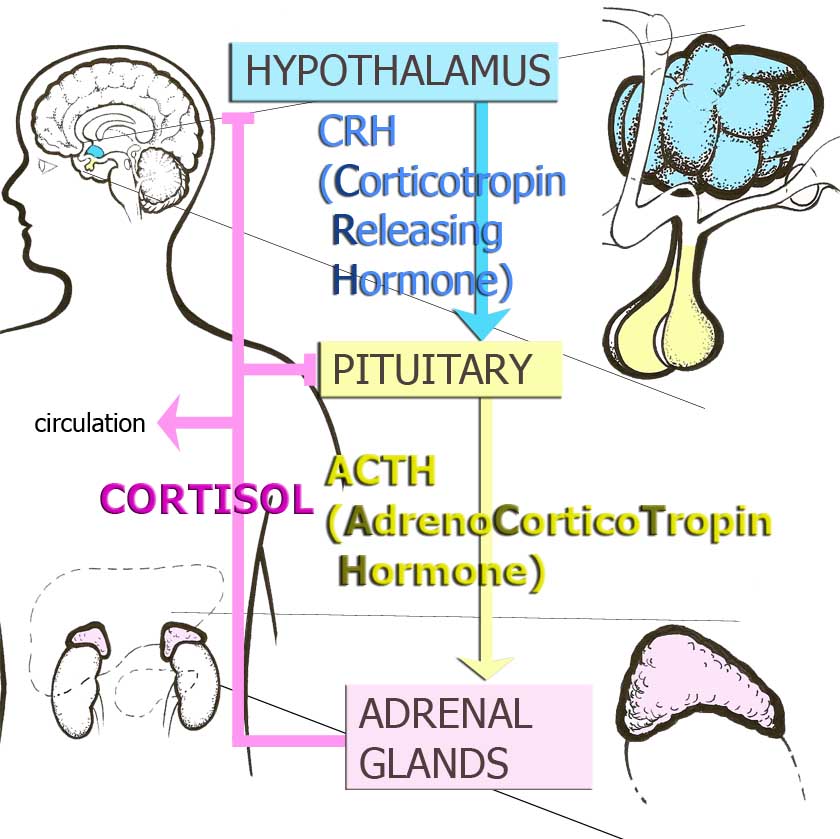
Figure 1. Hypothalamic-Pituitary Adrenocortical Axis or Stress Hormone Axis
The HPA axis maintains the organism’s capacity to respond to acute and prolonged stressors and is a major focus of ELS research, as the brain is a major organ targeted by steroid hormones produced by this system. In response to a stressor, the HPA axis becomes activated and the hypothalamus and other brain regions release corticotropin-releasing hormone (CRH).
CRH produced in the amygdala, a structure involved in orchestrating emotional responses, activates behavioural stress responses such as fight/flight responses, heightened vigilance, and defense-related learning and memory.3 CRH produced in the hypothalamus, a structure involved in maintaining homeostasis, stimulates production of adrenocorticotropin hormone (ACTH) by the pituitary gland, which then signals the cortex of the adrenal glands to produce and release cortisol (corticosterone in rodents). Cortisol facilitates adaptation and restores homeostasis through changing internal dynamics.
One problem for researchers studying stress in children is that, although the chronic effects of stress are often revealed by measures of ACTH and CRH,4 their measurement is invasive and not feasible with children.5 Therefore, most researchers rely on samples of cortisol obtained in saliva, which imposes certain limitations in explicating the regulation and dysregulation of this system. Additionally, researchers must consider that other factors affect cortisol levels such as time of day, child age, sleep/wake cycles, and social context. Nonetheless, research on this neuroendocrine system has burgeoned because of the use of salivary cortisol measures.
Another critical challenge is the complex and multifaceted nature of stress in childhood. Researchers must consider: (1) the type(s) of stressors the child faces, their chronicity and severity, (2) the family environment, (3) psychological mechanisms of coping and defense, (4) individual differences in reactivity, (5) and developmental status. The pathway from stress to psychopathology and/or medical problems likely involves many environmental factors, which continuously interact with an individual’s unique genetic code to shape HPA functioning and brain development.
Research Context
Examining the relationship between stress and brain development in humans relies on technology which has only recently become available, including imaging techniques to assess brain development and activity (e.g., structural and functional MRI, MEG, and so on), electrophysiological measures of brain activity, and more advanced and sophisticated techniques for measuring HPA axis functioning. These procedures have been used mostly in studies of the adult consequences of ELS. Only recently have researchers begun to examine the effects of ELS on child development. Here is where the scientific literature lags behind. Fortunately, animal models have played a critical role in helping researchers understand phenomena which has not yet been addressed or cannot be answered by studies of children. Findings in non-human primates and rodents6-11 have provided a framework by which researchers can formulate testable theories on the psychological and neurobiological impacts of stress in humans.
Key Research Questions
- What sources promote individual differences in how children respond to stressors?
- Which genetic and environmental factors protect children against the deleterious effects of ELS thus promoting resilience?
- What are the long-term consequences of ELS and are they reversible?
- What is the role of ELS in the development of psychopathology and medical health problems?
Recent Research Results
Research in humans increasingly suggests that severe early life stressors (e.g., trauma, maltreatment, neglect) may result in decreased brain volumes, dysregulation of the neuroendocrine stress response system, and limbic dysfunction involving regions such as the hippocampus, medial prefrontal cortex, and amygdala.12-18 Consistent with these findings, animal studies of severe ELS yield evidence of inhibition of neurogenesis, disruption of neuronal plasticity, neurotoxicity, and abnormal synaptic connectivity. Sensitive periods and stages of enhanced brain plasticity are particularly vulnerable to the long-term effects of stress hormones and may result in altering the typical pathways and organization of the young brain. Research also suggests that severe ELS may have mental and physical consequences that last into adulthood, including increased risk of depression, anxiety, post-traumatic stress disorder, metabolic syndrome, and cardiovascular disease.2,3,19-21
Notably, research has revealed that the child’s access to supportive, attentive, and sensitive adult care plays a salient role in buffering the activity of the HPA system and protecting the developing brain from potentially harmful effects of stressors.2,22-24 Children within secure parent-child relationships learn that when faced with a stressor, they can experience distress, communicate their negative emotions, and effectively elicit aid from caregivers. It is likely that this sense of safety prevents activation of the HPA axis and other critical stress-mediating systems.22-26
A small body of emerging literature suggests that the negative effects of stress are not always irreversible. Interventions which enhance the economic and emotional support of children undergoing considerable stress have been shown to improve both behavioural and emotional adjustment, and normative regulation of the HPA axis.27 Research has also found that behavioural therapy as well as drug therapy may bring about neurobiological changes in individuals suffering form the psychological effects of stress.28 Furthermore, there is increasing evidence that some experience with stressors early in life, particularly experiences that enhance the child’s capacity to cope effectively, may have stress inoculation effects. That is, they may decrease the reactivity of stress responsive neurobiological and neuroendocrine systems to stressors experienced later in life.29,30
Research Gaps
Most of the adult research on ELS relies on retrospective reports of ELS experiences. Prospective studies are needed to elucidate how the types of stressors children experience at different points in development impact the development of physiological and behavioural responses to subsequent challenges. Additionally, stress research has yet to elucidate the processes and mechanisms through which social support buffers against the harmful effects of stress. It is also unclear how childhood stress, in combination with concurrent psychopathology, differentially affects HPA axis regulation. Furthermore, neuroanatomical and neurophysiological studies are needed to more fully explicate ELS effects on specific brain structures and processes. Finally, although an active area of research, the field still lacks an adequate understanding of the genetic variations among children that moderate the reactivity, regulation, and impact of stress responses.
Conclusion
As children grow into mature adults, they will inevitably be faced with challenges, both predictable (e.g., beginning the first day of school) and unpredictable (e.g., the loss of a loved one). These challenges provide children with the opportunity to learn how to effectively manage stress, regulate emotions, and develop the social, behavioural, and cognitive coping resources needed to overcome these obstacles. The presence of sensitive and responsive caregivers can help equip children with the tools needed to handle stress in a healthy manner.
The early years of life constitute a particularly sensitive period during which chronic stress may lead to dysregulation of the stress system and may compromise brain development. Not all individuals are equally at-risk for developing neurobiological, behaviour and health consequences of ELS. It is likely that genetic factors, emotional and behavioural predispositions, stress history, social support, mental health status, age, and sex all play a role in stress reactivity and regulation. Tracing the pathways through which early adversity impacts later development is the key challenge for developmental stress research in the coming decade.
Implications
Although we do not yet have a full understanding of the neurobiological and neuroendocrine processes through which ELS affects development, the present state of the science is sufficient to draw implications for policy and practice. Many of these implications are outlined in a working paper on stress and brain architecture produced by the National Scientific Council on the Developing Child and available on the council’s website.a,31 These implications include: (1) The need to strengthen a range of informal and formal services to support parents who are struggling to provide care for their children; (2) The need to make affordable expert assistance available to parents and early child care professionals to equip them with the knowledge and skills to help children who have symptoms of abnormal stress responding before these problems produce pathology; (3) The need to increase the availability of assessment and treatment for young children with serious stress-related mental health problems; (4) And, because parental substance abuse and mental illness are associated with increased risk of toxic stress exposures for young children, these conditions and the economic circumstances associated with them are a major public health problem needing significant public attention.
References
- Heim C, Nemeroff CB. The role of childhood trauma in the neurobiology of mood and anxiety disorders: preclinical and clinical studies. Biological Psychiatry 2001;49(2):1023-1039.
- McEwen BS. Understanding the potency of stressful early life experiences on brain and body function. Metabolism 2008;57(Suppl 2):11-15.
- Heim C, Owen MJ, Plotsky PM, Nemeroff CB. The role of early adverse life events in the etiology of depression and posttraumatic stress disorder: Focus on corticotropin-releasing factor. Annals of the New York Academy of Sciences 1997; 821:194-207.
- Heim C, Newport DJ, Heit S, Graham YP, Wilcox M, Bonsail R, Miller AH, Nemeroff CB. Pituitary-adrenal and autonomic responses to stress in women after sexual and physical abuse in childhood. Journal of the American Medical Association 2000;284(5):592-597.
- Gunnar MR, Talge NM. Neuroendocrine measures in developmental research. In: Schmidt LA, Segalowitz S, eds. Developmental Psychophysiology: Theory, Systems, and Methods. New York: Cambridge University Press; 2008: 343-366.
- Francis D, Diorio J, Plotsky PM, Meaney MJ. Environmental enrichment reverses the effects of maternal separation on stress reactivity. Journal of Neuroscience 2002;22(18):7840-7843.
- Levine S, Wiener SG. Psychoendocrine aspects of mother-infant relationships in nonhuman primates. Psychoneuroendocrinology 1988;13(1-2):143-154.
- Sanchez MM, Noble PM, Lyon CK, Plotsky Davis M, Nemeroff CB, Winslow JT. Alterations in diurnal cortisol rhythm and acoustic startle response in nonhuman primates with adverse rearing. Biological Psychiatry 2005;57(4):373-381.
- Schneider ML, Moore CF. Effect of prenatal stress on development: A nonhuman primate model. In: Nelson CA, ed. Minnesota Symposium on Child Psychology. Mahwah, NJ: Lawrence Erlbaum Associates Publishers; 2000: 201-244. Vol 31: Effects of early adversity on neurobehavioral development.
- Smythe JW, McCormick CM, Rochford J, Meaney MJ. The interaction between prenatal stress and neonatal handling on nociceptive response latencies in male and female rats. Physiology and Behavior 1994;55(5):971-974.
- Suchecki D, Mazzafarian D, Gross G, Rosenfeld P, Levine S. Effects of maternal deprivation on the ACTH stress response in the infant rat. Neuroendocrinology 1993;57(2):204-212.
- Bremner J, Narayan M. The effects of stress on memory and the hippocampus throughout the life cycle: Implications for childhood development and aging. Development and Psychopathology 1998;10(4):871-885.
- De Bellis MD, Baum, AS, Birmaher B, Keshavan MS, Eccard CH, Boring AM, Jenkins FJ, Ryan ND. Developmental traumatology, Part 1: Biological stress systems. Biological Psychiatry 1999;45(10):1259-1270.
- Glaser D. Child abuse and neglect and the brain—a review. Journal of Child Psychology and Psychiatry 2000;41(1):97–116.
- Sapolsky, R. Why stress is bad for your brain. Science 1996;273(5276): 749-750.
- Teicher MH, Anderson SL, Dumont Y, Ito CA, Glod C, Vairuzis C, Giedd JN. Childhood neglect attenuates development of the corpus callosum. Paper presented at: The Annual Meeting of the Society for Neuroscience: November, 2000; New Orleans, LA .
- Teicher MH, Andersen SL, Polcari A, Anderson CM, Navalta CP. Developmental neurobiology of childhood stress and trauma. Psychiatric Clinics of North America. 2002;25(2):397-426.
- Tottenham NH, Hare TA, Quinn BT, McCarry TW, Nurse M, Galvan A, Davidson MC, Thomas KM, McEwen B, Gunnar M, Aronson J, Casey BJ. . Amygdala volume and sensitivity to emotional information following orphanage rearing. Journal of Child Psychology & Psychiatry. In press.
- Bremner JD, Vythilingam N, Vermeetn E, Adil J, Khan S, Nazeer A, Afzal N, McGlashan T, Elzinga B, Anderson GM, Heniger G, Southwick SM, Charney DS.. Cortisol response to a cognitive stress challenge in posttraumatic stress disorder (PTSD) related to childhood abuse. Psychoneuroendocrinology 2003;28(6):733–750.
- Heim C, Newport JD, Mletzko T, Miller AH, Nemeroff CB. The link between childhood trauma and depression: Insights from HPA axis studies in humans. Psychoneuroendocrinology 2008;33(6):693-710.
- Yehuda R, Halligan SL, Grossman R. Childhood trauma and risk for PTSD: relationship to intergenerational effects of trauma, parental PTSD, and cortisol excretion. Developmental Psychopathology. 2001;13(3):733-753.
- Gunnar MR. Quality of early care and buffering of neuroendocrine stress reactions: Potential effects on the developing human brain. Preventive Medicine: An International Journal Devoted to Practice and Theory. 1998;27(2):208-211.
- Gunnar MR, Donzella B. Social regulation of the cortisol levels in early human development. Psychoneuroendocrinology 2002;27(1-2):199-220.
- Gunnar MR, Larson M, Hertsgaard L, Harris M, Brodersen L. The stressfulness of separation among 9-month-old infants: effects of social context variables and infant temperament. Child Development 1992;63(2):290-303.
- Ahnert L, Gunnar MR, Lamb M, Barthel M. Transition to child care: associations with infant-mother attachment, infant negative emotion and cortisol elevations. Child Development 2004;75(3):639-650.
- Hertsgaard L, Gunnar MR, Erickson M, Nachmias M. Adrenocortical responses to the strange situation in infants with disorganized/disoriented attachment relationships. Child Development 1995;66(4):1100-1106.
- Fisher PA, Gunnar MR, Chamberlain P, Reid JB. Preventive intervention for maltreated preschool children: Impact on children's behavior, neuroendocrine activity, and foster parent functioning. Journal of the American Academy of Child and Adolescent Psychiatry. 2000;39(11):1356-1364.
- Baxter L, Schwartz J, Bergman K, et al. Caudate glucose metabolic rate changes with both drug and behavior therapy for obsessive-compulsive disorder. Archives of General Psychiatry 1992;49(9):681-689.
- Ellis BJ, Jackson JJ, Boyce WT. The stress response systems: Universality and adaptive individual differences. Developmental Review 2006;26(2):175-212.
- Lyons DM, Parker KJ. Stress inoculation-induced indications of resilience in monkeys. Journal of Traumatic Stress 2007;20(4):423-433.
- National Scientific Council on the Developing Child. Excessive Stress Disrupts the Architecture of the Developing Brain. Working Paper No.3; 2005. Available at: http://www.developingchild.net/pubs/wp/Stress_Disrupts_Architecture_Developing_Brain.pdf. Accessed December 18, 2008.
Note:
a See also the National Scientific Council on Child Development Publications available at: http://www.developingchild.net/pubs/wp.html Accessed February 13, 2009.
How to cite this article:
Gunnar MR, Herrera A, Hostinar CE. Stress and Early Brain Development. In: Tremblay RE, Boivin M, Peters RDeV, eds. Encyclopedia on Early Childhood Development [online]. https://www.child-encyclopedia.com/brain/according-experts/stress-and-early-brain-development. Published: June 2009. Accessed February 27, 2026.
Text copied to the clipboard ✓